|
Geological Survey Circular 838
Guides to Some Volcanic Terrances in Washington, Idaho, Oregon, and Northern California |
MECHANISM OF MAGMA MIXING AT GLASS MOUNTAIN, MEDICINE LAKE HIGHLAND VOLCANO, CALIFORNIA
John C. Eichelberger
Geosciences Division, University of California,
Los Alamos Scientific Laboratory, Los Alamos, NM 87545
ABSTRACT
Mixing of basaltic and rhyolitic magmas at Glass Mountain appears to have been driven by vesiculation of basaltic magma as it intruded a rhyolitic magma chamber. Rapid cooling of basaltic magma formed a mafic foam which floated and became concentrated at the roof of the chamber. Foam-rich lava emerged first during the eruption and became the hybrid dacite of the distal end of the flow. The chamber is probably a relatively large volume, long-lived fea ture, lying within 10 km of the surface beneath the caldera. Eruption of similar lava at Crater Lake shortly before caldera collapse supports this interpretation.
This mechanism of mixing between silicic magma stored in a crustal chamber and basaltic magma feeding the chamber is controlled by initial water content of basaltic magma, and implies that dry basaltic magma would remain at the base of the chamber. The eastward change from andesitic to bimodal volcanism in this portion of the Cascade Range may be due to an eastward decrease in water content of parental basaltic magmas.
INTRODUCTION
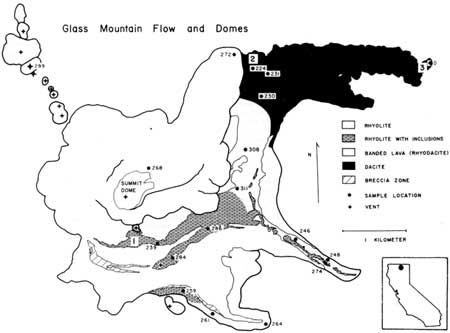
The largest and most imposing product of Holocene activity at the Medicine Lake Highland Volcano is the Glass Mountain lava flow (Figure 1). The most perplexing feature of this flow is its lithologic variety. The 1 km3 of lava which comprises the flow ranges from dull, stony, porphyritic dacite charged with fine-grained mafic xenoliths to shiny, black, phenocryst-free and xenolith-free obsidian. The range in silica content is from 66 wt.% to 73 wt. %.
Anderson (1933) described the geologic setting and general petrologic features of the Glass Mountain flow. Additional data on its age and history were provided by Finch (1928), Anderson (1941), Chesterman (1955), and Friedman (1965). More recently, Condie and Hayslip (1974) and Mertzman (1977) presented geochemical and petrologic data for Highland lavas, and I (Eichelberger, 1975) studied the compositional zonation of the Glass Mountain flow as a means for understanding relationships among magmas of the basalt-andesite-dacite-rhyolite family. Mapping and sampling of lava streams within the flow revealed that the eruption was continuous and, in general, proceeded from homogeneous dacite through highly heterogeneous banded rhyodacite to rhyolite. This suggested a compositional zonation of the parent magma body in which dacitic magma graded downward to rhyolitic magma. Electron microanalysis of phases within the dacite indicated that most of the phenocrysts. An75 plagioclase, Fo80 olivine and Wo41 En43 FS16 augite, were derived from disaggregation of the crystal-rich basaltic xenoliths. The dark dacitic bands of the heterogeneous rhyodacite are streams of basaltic debris from disintegrating xenoliths. This process was more advanced in the dacite into which the rhyodacite grades, yielding an intermediate magma that was megascopically homogeneous except for residual xenoliths. Since this hybrid magma was concentrated at the top of the chamber, and since the Highland shield through which the magma passed contains a high proportion of mafic lava flows and cinder cones, it was concluded that mafic material had sloughed off the roof of the magma chamber and contaminated the top of the magma body. However, in subsequent studies of lavas of similar composition from other Cascade volcanoes, I found similar xenoliths and recognized evidence that the xenoliths were liquid when they came in contact with silicic magma (Eichelberger, 1978). This evidence includes:
l. Rounded shape of xenoliths.
2. Texture indicating rapid crystallization to just above solidus.
3. Incorporation of phenocrysts from the host silicic magma in rinds on the outer portions of some large xenoliths.
4. Outward decrease in grain size in some large xenoliths.
5. Reaction or resorption of phenocrysts in the host lava indicating heating of the silicic magma during mixing.
Although the parental rhyolitic magma of Glass Mountain lacked any phenocrysts to record a thermal event associated with mixing, significant crystallization should have occurred if cool rock had been stirred into the chamber. Lack of such crystallization suggested that mixing at Glass Mountain might have involved two magmas rather than magma and rock, and that a reevaluation of the data was in order.
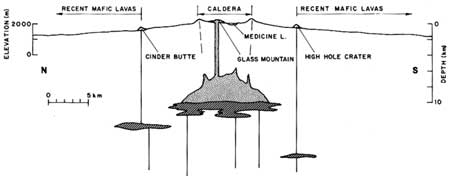
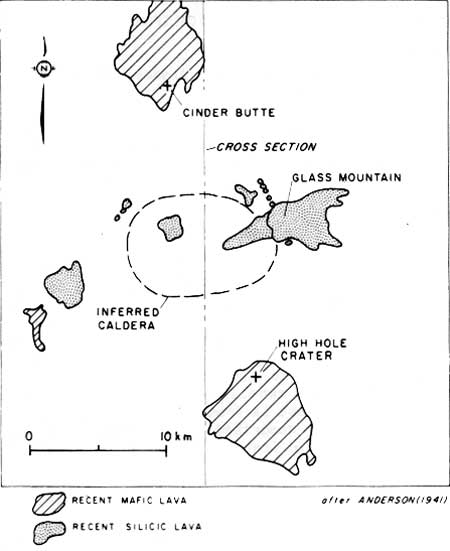
The arrangement of vents on the Highland, silicic vents associated with a caldera structure and flanked by numerous mafic vents (Figure 2), raises the possibility that mafic magma is supplying a shallow silicic chamber (Figure 3). However, such a mechanism would introduce mafic magma to the base of the chamber and would seem to produce a compositional zonation opposite to that observed in the Glass Mountain flow. Evidence that mixing occurs by floatation of basaltic foam in silicic chambers (Eichelberger, 1979) affords a solution to this problem and provides a basis for understanding the evolution of the Medicine Lake magma chamber.
|
| Figure 2. Map showing distribution of Holocene lavas of the Medicine Lake Highland. |
VAPOR EXSOLUTION DURING MIXING
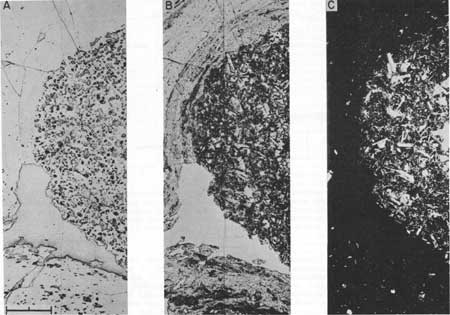
Mixing of large batches of magma requires flow on a large scale within a magma chamber. A likely mechanism of large scale flow and stirring is convection. Introduction of mafic magma into the base of a chamber would strongly heat silicic magma in the lower portion of the chamber and should induce relatively rapid convection (Rice and Eichelberger, 1976; Sparks and others, 1977). But because the density of basalt is high, any mixing of heated silicic magma with basaltic material would inhibit convection. For example, the density decrease due to heating silicic magma by 100°C could be offset by addition of only 2 volume percent basaltic material. The system would probably remain stratified, with the silicic and basaltic layers convecting separately, and mixing limited to diffusion at the interface. Of course, if the basaltic material were less dense than the silicic magma, heating of silicic magma and mixing with basaltic material would both decrease density of magma in the lower portion of the silicic layer, resulting in strong upward flow. Despite the high density of phases crystallized by basaltic magma and present in abundance in the basaltic xenoliths contained in andesitic and dacitic lavas, it appears that the bulk density of these xenoliths is actually less than that of the lavas in which they occur. The reason for this apparent contradiction is that the xenoliths invariably contain abundant, fine, round, uniformly distributed vesicles. Although vesiculation can occur at very shallow depth during eruption, these vesicles may be of deep, primary origin. As stated before, the texture of the xenoliths suggests rapid crystallization of basaltic melt in contact with cooler silicic magma. During the six hours required for a chill wave to propagate 10 cm into basaltic magma, corresponding to roughly the largest common diameter of the xenoliths, a hydration front could advance only 10-2 cm into the silicic magma, based on data of Shaw (1974). Thus, heat is extracted from the basalt orders of magnitude faster than water, so that the basalt behaves nearly as a closed system with respect to water. Under low to moderate crustal pressures a vapor phase exsolves because water is contained entirely within the melt phase of the system and the melt fraction decreases with crystallization from almost 1.0 to about 0.1. The result is a basaltic foam layer at the base of the silicic magma body that can float and mix. Floatation of the foam exposes fresh basaltic magma to cool silicic magma, so that the process of foam formation continues. Dispersal of foam through the silicic magma is due to convective flow, driven both by thermal expansion and the positive AV phase change. Photomicrographs showing the Glass Mountain xenoliths to be a crystal-rich foam are presented in Figure 4.
|
| Figure 4. Photomosaics of basaltic xenolith in banded rhyodacite lava from Glass Mountain. Views are reflected light (A). transmitted light (B), and transmitted light with crossed polars (C). Bar length is 1.0 mm. Note abundant, 0.1 mm-diameter vesicles in zenolith (A) and absence of vesicles in most of the host lava, except for a large vesicle at the lower left edge of the xenolith. Lava at the bottom is contaminated with basaltic material and contains some vesicles. In B, the zenolith is a dark round object with prominent white plagioclase crystals. Pyroxene is also a major phase, with olivine. asides, and glass present in lesser amounts. The large vesicle associated with the zenolith is an irregular white area. Lava contaminated with basaltic material shows up as dark streaks, In C, the crystal-rich character of the xenolith is apparent. Contaminated lava contains more crystals than uncontaminated lava. |
DENSITY MEASUREMENTS AT GLASS MOUNTAIN
To test the hypothesis that mixing is driven by vapor exsolution, samples of Glass Mountain lava and xenoliths were collected for density measurements. Several pairs of lava and xenolith samples were collected from sites (Figure 1) near the vent, middle, and distal end of the longest lava stream, representing "rhyolite with inclusions," dacite near the transition to rhyodacite, and the most mafic dacite lava of the flow, respectively (Eichelberger, 1975). Density was determined by measuring dry and submerged weight of samples which ranged in size from 300 g to 600 g. Results are given in Table 1. The obsidian near the vent has no porosity and a very narrow range of density (smaller than analytical error), 2.38±0.01 g/cm3. Dacite from midway down the stream is 2.49 g/cm3. Dacite from the distal end is 2.55 g/cm3, reflecting its more mafic and crystal-rich character. Bulk density of the xenoliths fall in a surprisingly narrow range. 2.15±0.04 g/cm3. This density appears little affected by the vesicularity of the host lava, since a xenolith in a pumiceous (1.49 g/cm3) sample has a density of 2.10 g/cm3, In order to determine the mass density of xenolith material, xenolith samples were ground to a fine sand to destroy the vesicles. Grain size of the sand averaged 100 µm and ranged from 1 µm to 400 µm. Examination of polished grain mounts revealed that grinding opened at least 99% of the pores. The sand was then weighed dry and submerged. The submerged measurement was made after the sample was subjected to a vacuum for 20 minutes, and water added under vacuum. The resulting density of 2.79±0.01 g/cm3 was expected for this predominantly plagioclase and pyroxene material. Using the average values for bulk and mass density gives a porosity of 23%. Thus the zenolithic material is much denser than the lava, but because of high porosity, the xenoliths would have floated in the magma chamber. This explains why the most zenolith-rich lava emerged first.
CONDITIONS OF MIXING
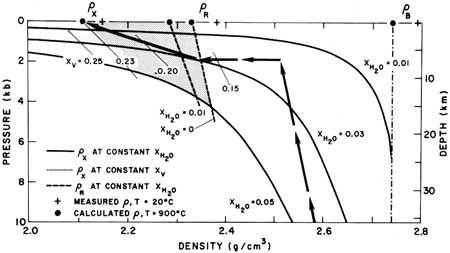
In view of the evidence that the xenoliths floated, and because densities as a function of pressure and temperature of the materials involved are known or approximately known, constraints can be placed on the depth of the magma chamber (Figure 5). Following previous definitions (Eichelberger, 1979), pB and pX are the mass and bulk densities of the basaltic xenoliths, so that pX = pB (1-XV) + pVXV, where pV and XV are the density and volume fraction of the vapor phase, respectively. Bulk density of the foam (pX) versus pressure was calculated at T = 900°C for bulk water content in basaltic magma of 1%, 3%, and 5%, by weight, using the following assumptions:
1. The vapor phase is water.
2. The zenolithic material (exclusive of vapor) is 10% melt and 90% crystals. This estimate is based on inspection of thin sections of xenoliths (grain size is too small for accurate modal analysis) and is consistent with the observed mass density, In the general case, degree of crystallization is dependent largely on temperature contrast between the basaltic and silicic magmas.
3. The zenolithic material is incompressible, In fact, the melt becomes less dense with increasing pressure because more water is retained and plagioclase and pyroxene compress, but the effects are small, opposite and nearly equal.
The measured value of pV was used, correcting for thermal expansion of plagioclase and pyrozene (Skinner, 1966). Data of Burnham and Jahns (1962) were used for solubility of water in melt, and data of Burnham and others (1969) were used for density of the vapor phase (pV). The resulting curves (Figure 5) show the density of the foam formed during mixing at a specified pressure and water content of basaltic magma. Portions of pX versus pressure curves for constant porosity of foam are also shown.
Density of magma in the chamber before mixing can be estimated for elevated temperature and pressure by assuming that rhyolitic glass and melt behave in a manner similar to glass and melt in the system albite + water (Burnham and Davis, 1971). The petrologic data indicate that magma in the chamber was entirely rhyolitic melt. Hence the density measurements for rhyolite obsidian were used. Water content strongly affects both thermal expansion and compressibility of rhyolite glass and melt. Available analyses give water contents of Glass Mountain obsidian of a few tenths of a percent (Anderson, 1941). Therefore, pB versus pressure at 900°C was calculated for 0 and 1 wt.% water. Knowledge that the xenoliths floated constrains xenolith density to those portions of the pX curves left (lower density) of the dry pB curve. Estimates of water content for high alumina basaltic magma of the type involved in sizing range upward to 5 wt.% (Anderson. 1974; Rose and others, 1978). This constrains pressure to a region above (lower pres sure) the 5 wt.% water pX curve. Since the xenoliths have most likely experienced continuously decreasing pressure since formation, it is unlikely that they ever possessed higher than their present (surface) porosity (XV), which constrains xenolith density to the right (higher density) of the XV = 0.23 curve. These constraints bound the shaded region in Figure 5. If we knew that the basalt initially contained 3 wt. % water, which is probably more reasonable since Medicine Lake basalt eruptions are much less gaseous than the Fuego eruptions for which the 5 wt. % water estimate was made, then the possible region would be a small triangle bounded below by the pX curve for 3 wt. % H2O, to the right by the appropriate pB curve, and above by a horizontal line extending from the intersection of the pX curves for 3 wt. % H2O and 23% porosity (foam with XH2O = 0.03 above this line would have XV >0.23). Regrettably, water content of basaltic magma is a major unknown, but it seems likely from these considerations that the Glass Mountain magma chamber lies within 15 km and probably within 10 km of the surface.
TABLE 1. Densities of Glass Mountain Samples
| Sample (Fig. 1) |
Bulk Density of Xenolith (g/cm3) |
Density of host lava (g/cm3) |
Sample Description |
| 1A | 2.14 | 2.40 | Rhyolite obsidian |
| 1B | 2.13 | 2.37 | Rhyolite obsidian |
| lB | 2.14 | 2.37 | Rhyolite obsidian |
| 1C | 2.20 | 2.38 | Rhyolite obsidian |
| 1D | 2.18 | 2.39 | Rhyolite obsidian |
| 1E | 2.10 | 1.49 | Rhyolite Pumice |
| 2A | 2.10 | 2.49 | Nonvesicular dacite |
| 2B | 2.23 | 2.49 | Nonvesicular dacite |
| 3B | 2.15 | 2.25 | Vesicular dacite |
| 3B | 2.13 | 2.42 | Vesicular dacite |
| 3B | 2.ll | 2.55 | Nonvesicular dacite |
| Averages | 2.15+0.04 | 2.38±0.01 | Rhyolite obsidian |
| 2.49 | Nonvesicular dacite, site #2 | ||
| 2.55 | Nonvesicular dacite, site #3 | ||
| Sample | Mas Density, Xenolith (g/cm3) | ||
| 1B | 2.79 | ||
| 1D | 2.79 | ||
| 2A | 2.78 | ||
| Average | 2.79±0.01 XV = 0.23 | ||
|
| Figure 5. Density versus pressure (depth) for materials from Glass Mountain. pX is bulk density of basaltic foam (xenoliths), pR is density of the reservoir magma, and pB is mass density of zenolithic material. The possible region for the Glass Mountain magma chamber is shaded. Arrows show path for ascending basaltic magma with 3 wt. % water. See text for further discussion. |
A possible path of ascending basaltic magma is shown as arrows in Figure 5. assuming XH2O = 0.03 for basalt and XH2O = 0 to 0.01 for rhyolite. The basalt ascends through the crust, slowly becoming less dense as indicated by the steep path from 10 kb to 2 kb. Densities along this path were calculated using average Medicine Lake basalt (Anderson, 1941) and the model of Bottinga and Weill (1970). Note that at 2 kb the magma is still vapor undersaturated. However, at this depth it enters the Glass Mountain magma chamber, undergoes rapid crystallization while exsolving a vapor phase, and forms a foam with pB = 2.74 g/cm3, XV >=0.17, and pX <=2.34 g/cm3. If this foam remained rigid but leaked after formation it would follow a constant porosity curve to the surface. If it maintained an equilibrium pore pressure and did not leak, it would follow a constant water curve and blow up during ascent. The path shown is intermediate: some leakage and some expansion to the observed XV = 0.23. The Glass Mountain xenoliths probably did leak, since most xenoliths have a large vesicle at the lava/xenolith interface, as shown in Figure 4. Inflation of the foam does work, energy for which is supplied largely by crystallization. At 2 kb, energy available from crystallization is nearly two orders of magnitude greater than energy required for inflation.
THE GLASS MOUNTAIN ERUPTION
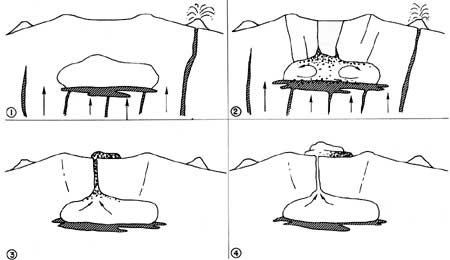
Important implications of this discussion for Glass Mountain are that water played an important role in magma evolution and that the chamber lies at shallow depth. A probable reconstruction of the eruption sequence is shown in Figure 6. Basaltic magma intruded the chamber and formed a foam which floated and mixed (in a mechanical sense) with magma in the chamber. The subsequent eruption first tapped the hybrid which was concentrated at the top of the chamber. Evidence that vapor exsolution occurred within the chamber suggests that mixing triggered the eruption. Assuming that 0.2 km3 of the flow is a 1:3 mixture of basalt and rhyolite then the volume of vapor exsolved within the chamber was 0.01 km3. Some of this almost certainly leaked from the foam and dissolved in the strongly water undersaturated rhyolitic melt. Nevertheless, a rapid volume increase within the chamber could most easily be accommodated by extrusion of material from the chamber. Thus, the mixing and eruption events were probably closely spaced in time, and may correlate with the extrusion of mafic magma 10 km to the south on the flanks of the Highland at High Hole Crater and its associated Burnt Lava Flow. Similar rhyolite and hybrid dacite have erupted before near Glass Mountain and elsewhere near the susmit of the Highland. These may be products of repeated intrusion of basaltic magma into a relatively large. shallow, long-lived magma chamber beneath the caldera (Figure 3). This would account for the liquidus or superheated condition of some of these lavas, as indicated by their lack of phenocrysts.
|
| Figure 6. Probable eruption sequence at Glass Mountain. (1) Mafic magma intrudes shallow rhyolitic magma chamber and erupts nearby at High Hole Crater. (2) Vesiculation of chilled mafic magma forms foam which floats and mixes in the convecting chamber. (3) Vesiculation in chamber triggers eruption, which first taps hybrid dacite concentrated near roof of chamber. (4) Eruption continues with extrusion of rhyolite magma. Rhyolitic tephra which underlies Glass Mountain may have erupted early in this sequence, as an immediate response to intrusion of mafic magma into the chamber, before mixing was far advanced. Alternatively, low density of water-rich rhyolitic magma at the top of the chamber say have prevented further rise of foam-rich currents. so that a small rhyolitic cap remained near the chamber roof and erupted as tephra just before the hybrid dacite. |
Although water content of basaltic magma is a major unknown, the high initial density contrast between basaltic and reservoir magmas (Eichelberger, 1979) constrains the Glass Mountain chamber to shallow depth. Both silicic composition and scarcity of phenocrysts in the reservoir magma contribute to this high density contrast. A remarkably similar high density contrast mixture erupted from Mt. Mazama to form the Llao Rock dacite flow, just prior to the Crater Lake event. The subsequent climactic tephra eruption shows that Mt. Mazama overlay a large shallow reservoir containing crystal-poor silicic magma, with mafic magma at the base of the reservoir. The Glass Mountain data suggest that this condition now exists beneath the Medicine Lake Highland.
Another consequence of this hypothesis is that mixing between magma stored in a crustal reservoir and mafic magma feeding the reservoir is controlled by the water content of the mafic magma. Mixing during replenishment of crustal magma reservoirs offsets compositional effects of fractionation or crustal melting, so that eruption products of reservoirs fed by wet mafic magma will tend to be intermediate in composition. Volcanism resulting from dry mafic magma will be bimodal because mafic magma remains at the base of the reservoir without mixing. Anderson (1974) suggested that Medicine Lake basalts are drier than those to the west of Mt. Shasta. This may account for the bimodal character of volcanism east of the High Cascades, farther from the plate boundary and the source of water.
ACKNOWLEDGMENTS
This work was supported by the Office of Basic Energy Sciences, U.S. Department of Energy. David Mann prepared the thin section shown in Figure 4.
REFERENCES CITED
Anderson, A. T., Jr., 1974. The before-eruption water content of some high-alumina magmas: Bull. Volcanol., V. 37, p. 530-552.
Anderson, C. A., 1933, Volcanic history of Glass Mountain, northern California: Am. Jour. Sci., V. 226, p. 485-506.
Anderson, C. A., 1941, Volcanoes of the Medicine Lake Highland, California: Univ. California Pubs. Geol. Sci., V. 25, p. 347-422.
Bottinga. Y. and Weill, D. F., 1970, Densities of liquid silicate systems calculated from partial molar volumes of oxide components: Am. Jour. Sci., V. 269, p. 169-182.
Burnham, C. W. and Davis, N. F., 1971, The role of H2O in silicate melts: I. P-V-T relations in the system NaAlSi3O8 - H2O to 10 kilobars and 1000°C: Am. Jour. Sci., V. 270, p. 54-79.
Burnham, C. W., Holloway, J. R., and Davis, N. F., 1969. Thermodynamic properties of water to 1000°C and 10,000 bars: Geol. Soc. Amer. Spec. Paper 132, p. 1-96.
Burnham, C. W. and Jahns, R. H., 1962, A method for determining the solubility of water in silicate melts: Am. Jour. Sci., V. 260, p. 721-745.
Chesterman, C. W., 1955, Age of the obsidian flow at Glass Mountain, Siskiyou County, California: Am. Jour. Sci., V. 253, p. 418-424.
Condie, K. C. and Hayslip D. L., 1975, Young bimodal volcanism at Medicine Lake volcanic center, northern California: Geochim. Cosmochim. Acta, V. 39, p. 1165-1178.
Eichelberger, J. C., 1975. Origin of andesite and dacite: Evidence of mixing at Glass Mountain in California and at other circum-Pacific volcanoes: Geol. Soc. Amer. Bull., V. 86, p. 1381-1391.
Eichelberger, J. C., 1978. Andesitic volcanism and crustal evolution: Nature, V. 275, p. 21-27.
Eichelberger, J. C., 1979, Vapor exsolution and mixing during replenishment of crustal magma reservoirs: Submitted to Nature.
Finch, R. H., 1928, Lassen report No. 14. Volcano Letter, V. 161, p. 1.
Friedman, I., 1968, Hydration rind dates rhyolite flows: Science, V. 159, p. 878-880.
Heiken, G., 1978, Plinian-type eruptions in the Medicine Lake Highland, California, and the nature of the underlying magma: Jour. Volcanol. Geothermal Res., V. 4, p. 375-402.
Mertzman, S. A., Jr., 1977, The petrology and geochemistry of the Medicine Lake Volcano, California: Contrib. Mineral. Petrol., V. 62, p. 221-247.
Rice, A. and Eichelberger, J. C., 1976, Convection in rhyolite magma (abstract): EOS. V. 57, p. 1024.
Rose, W. I., Jr., Anderson, A. T., Jr., Woodruff, L. G., and Bonis, S. B., 1978, The October 1974 basaltic tephra from Fuego Volcano: Description and history of the magma body: Jour. Volcanol. Geothermal Res., V. 4, p. 3-54.
Shaw, H. R., 1974, Diffusion of H2O in granitic liquids: Part I. Experimental data; Part II. Mass transfer in magma chambers: in Hofman, A. W.. Giletti, B. J., Yoder, H. S., Jr., and Yund, R. A., eds., Geochemical transport and kinetics. Carnegie Institution of Washington, V. 634, p. 139-170.
Skinner, B. J., 1966. Thermal expansion: in Clark, S. P., Jr., ed., Handbook of Physical Constants. Geol. Sco. Amer. Mem., V. 97, p. 78-96.
Sparks, R. J. S., Sigurdsson, H., and Wilson, L., 1977, Magma mixing: A mechanism for triggering acid explosive eruptions: Nature, V. 267, p. 315-318.
| <<< Previous | <<< Contents >>> |
circ/838/sec10.htm
Last Updated: 28-Mar-2006