|
Glacier
Ecology of the Rocky Mountain Goat in Glacier National Park and the Swan Mountains, Montana Douglas H. Chadwick |

|
ECOLOGY OF THE ROCKY MOUNTAIN GOAT
IN GLACIER NATIONAL PARK AND
THE SWAN MOUNTAINS, MONTANA
FINAL REPORT
DOUGLAS H. CHADWICK
1977
Glacier National Park
West Glacier, Montana 59936
Present address: P.O. Box 175
Polebridge, Montana 59928
ABSTRACT
Data are presented which illustrate that the nature of the terrain and food supply in mountain goat (Oreamnos americanus) habitat ultimately regulate mountain goat population characteristics. Individuals in small groups apparently possess only a slight selective advantage over solitary animals but a more substantial advantage over members of large groups. In certain respects, mountain goats are closer to a semi-gregarious species than a typical herd animal. This is expected for a species with a patchy food resource and dense cover, cover being in the form of topography rather than vegetation. Recurrently intense resource competition and movement patterns necessary to exploit shifting forage supplies have led to organization of herds by a dominance hierarchy rather than a territorial system. Aggressive intolerance, expressed through high rates of agonistic interaction, appears to be the primary mechanism limiting group sizes and dispersing groups within ranges. Within successionally stable plant communities, mountain goat populations appear to be regulated through density-related mortality in subordinate classes commensurate with climatically induced resource shortages.
Chadwick, D. H. 1977. Ecology of the Rocky Mountain goat in Glacier National Park and the Swan Mountains, Montana: final report. Glacier National Park, West Glacier, Montana. 54pp.
TABLE OF CONTENTS
Habitat Selection
Food Habits
Range
General Considerations
Glacier Park
Swan Mountains
Productivity
Mortality
Grouping
Group Size
Group Composition
Distribution Within Habitats
Leadership
Agonistic and Sexual Relationships
INTRODUCTION
It has traditionally been said of the Rocky Mountain goat that because of the exclusive nature of the precipitous terrain it inhabits, it is the one native ungulate species whose populations have not been significantly altered by the course of human development of western North America. This statement is no longer applicable. Mountain goat populations have experienced recent declines throughout much of their range in the face of increased accessibility and shooting pressures. Proper management is hindered by inadequate knowledge of the species' biology and by difficulties in obtaining reliable population estimates in rugged, hazardous terrain.
This paper defines ecological parameters and examines the natural regulation of mountain goat populations. Data have been drawn from ongoing studies of mountain goats on two native western Montana ranges. The two sets of data are directly comparable in some respects and complementary in others. They have been combined in order to provide a more complete picture of the species. It was also important to check results obtained in the Swan Mountains, where wildlife populations were subject to exploitation and habitat alteration by humans, with results from a relatively undisturbed ecosystem, Glacier National Park, which is designated and managed as a natural area.
I am indebted to the National Park Service, which supported my research while I was employed as a seasonal biologist at Glacier Park. Particular appreciation is extended to C. J. Martinka, Glacier Park Supervisory Research Biologist, for encouragement, direction, and sound scientific advice, and to K. L. McArthur for editing the manuscript. Other park personnel and Karen B. Reeves provided valuable assistance during my work in the park. Studies in the Swan Mountains were made possible by support from the Montana Fish and Game Department, the National Wildlife Federation, Defenders of Wildlife, and the University of Montana School of Forestry. I also wish to acknowledge the assistance of Beth Chadwick, Robert R. Ream, and U.S. Forest Service personnel during that phase of research.
STUDY AREAS
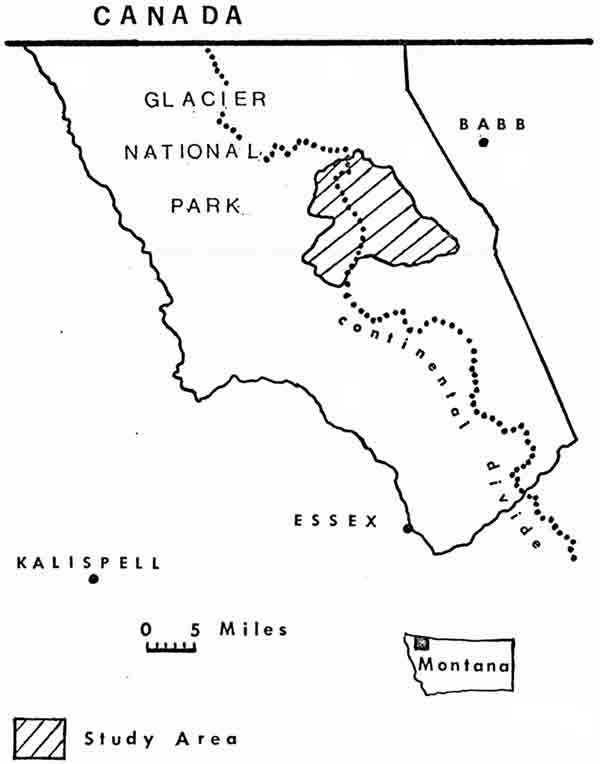
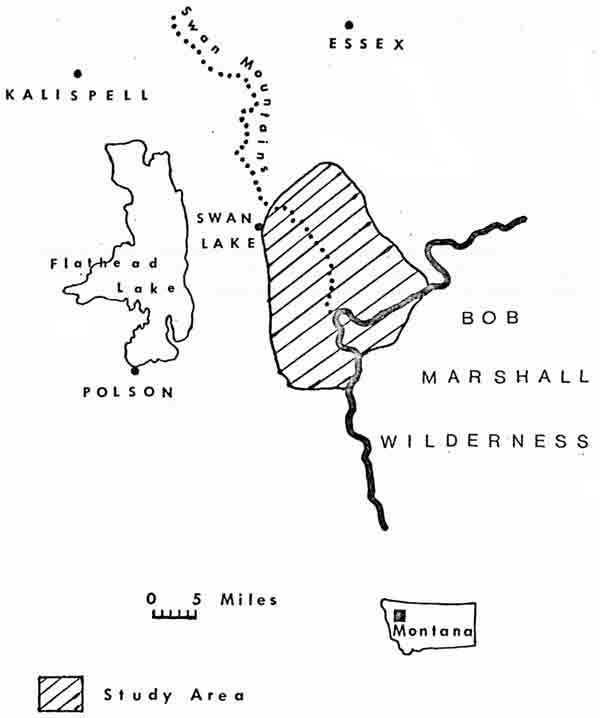
The two study areas were both located in northwestern Montana, about 125 km apart. The 310-km study area in the central region of Glacier Park lies on both sides of the Continental Divide and encompasses portions of 10 major drainages (Fig. 1). The 500-km2 Swan Mountains study area is situated at the northwestern corner of the Bob Marshall Wilderness in Flathead National Forest, west of the Continental Divide (Fig. 2), and was described in an earlier report (Chadwick 1974).
|
| Fig. 1. Location of Glacier National Park study area. |
|
| Fig. 2. Location of Swan Mountains study area. |
The Swan Mountains and western slopes of Glacier Park are subject to Pacific maritime weather influences which result in moderate temperatures and high precipitation, mostly in the form of snow. Air masses moving eastward lose most of their moisture on western slopes and bring frequent strong, drying winds to eastern slopes. Continental weather systems east of the Divide in the park result in colder, drier conditions there. Light winter conditions prevailed in 1972-73 and 1975-76, and 1974-75 was moderately severe.
Precambrian Belt Series argillites and limestones underlie both study areas in broad bedding planes which dip at moderate angles. Exposed sedimentary layers typically form steplike ledges and terraces. The major erosional agent has been repeated glaciation which has sculpted landforms dominated by sharp peaks and ridges, cirque basins, and wide U-shaped valleys. Present-day glaciers reappeared 4,000 years ago (Ross 1959) and remain active erosional agents both in the park and, to a lesser extent, in the Swan Mountains. Recent glacial fluctuations were described by Matthes (1942) and Dyson (1949).
Elevations in both study areas range from 1,060 m to 3,050 m. Eastern park slopes average 300 m higher than western slopes. Just over one-third of the entire park and one-half of the study area lie with in the alpine zone. Only about one-tenth of the Swan Mountains and the representative study area may be characterized as alpine. The "alpine zone" is typified by persistent snowpack, extreme low temperatures, extensive frost action, high wind velocities, and uniquely adapted, slow-growing plant communities.
Upper elevations in both study areas are dominated by sparsely vegetated rock outcroppings with fellfield communities developing at their bases. Also prominent at high elevations are tundra communities, characterized by mat-forming forbs, and meadows dominated by sedges (Carex spp.) or rushes (Luzula spp.). With increasing soil establishment, krummholz subalpine fir (Abies lasiocarpa) and whitebark pine (Pinus albicaulis) communities begin at about 2,130 m and grade into typical spruce (Picea engelmannii) — subalpine fir forests near 1,520 m. Beargrass (Xerophyllum tenax) is a prominent under-story species of both forest types. Bunchgrass communities of Agropyron spicatum, Calamagrostis rubescens, and Poa spp. are found on ledges with sufficient soil development, on dry open hillsides, and on well-drained bottomlands.
Frequent, often catastrophic, downslope movement of snow, ice, rocks, and water maintains widespread seral vegetation on precipitous slopes. Recent fires have disturbed some portions of goat winter ranges but do not appear to be a major influence. Alder (Alnus sinuata), menziesia (Menziesia ferruginea), and various willows (Salix spp.) are the most common seral vegetation on moist slopes, while shrubfields of mountain maple (Acer glabrum), serviceberry (Amelanchier alnifolia), and mountain ash (Sorbus americanus) dominate seral vegetation on drier slopes. More detailed descriptions of vegetation of the Swan Mountains and Glacier Park can be found in Chadwick (1974) and Habeck (1970), respectively.
Wolves (Canis lupus), coyotes (Canis latrans), wolverine (Gulo gulo), cougar (Felis concolor), lynx (Lynx canadensis), grizzly bears (Ursus arctos), black bears (U. americanus), golden eagles (Aquila chrysaetos), and bald eagles (Haliaeetus leucocephalus) were observed within mountain goat range in both study areas. Artiodactyls present included elk (Cervus elaphus), mule deer (Odocoileus hemionus), and rarely moose (Alces alces) and white-tailed deer (O. virginuanus). Bighorn sheep (Ovis canadensis) occurred on eastern slopes in Glacier Park. Predator and ungulate populations in Glacier Park were protected, and most were assumed to be naturally distributed and abundant. Distribution and abundance of wildlife populations in the Swan Mountains may have reflected past and present shooting and trapping, increased human access through roadbuilding, and habitat alteration related to logging activities.
METHODS
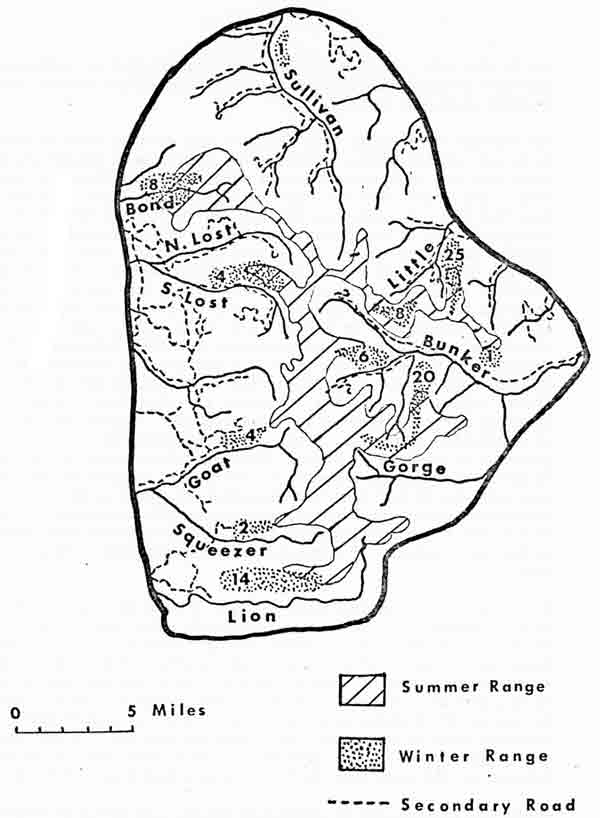
Research in the Swan Mountains focused on a single herd of 25-35 animals in the Bunker and Little Creek drainages (Fig. 3) during 1971-74. This herd was observed on a daily basis for periods of 18 continuous months, 7 continuous months, and numerous shorter intervals. Detailed maps were constructed of 2 key ranges, and daily movements and activities of individual goats were charted on them. Fourteen goats were marked: 5 with radiotransmitters (after Ream et al. 1971), 5 with nylon collars (after Craighead et al. 1969), and 4 with dye. Patches of dye were transferred to subjects by Cap-Chur projectile darts or automatic mechanisms placed along heavily used goat trails.
|
| Fig. 3. Location of summer and winter mountain goat ranges in the Swan Mountains study area. Numbers indicate approximate numbers of goats present on winter ranges in 1973. |
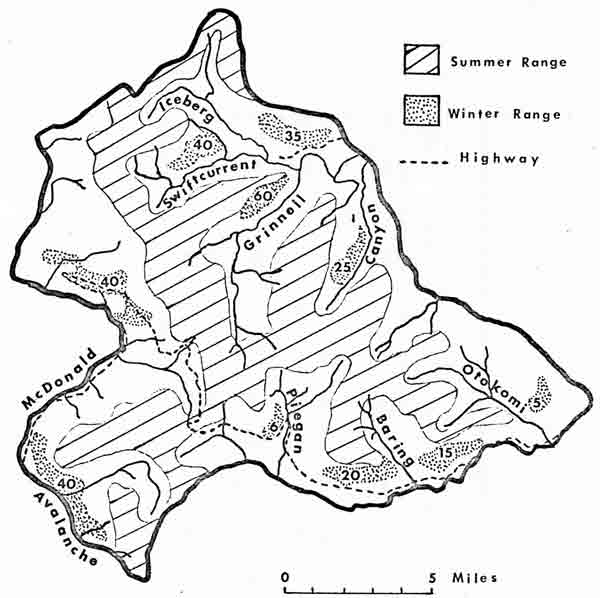
The Glacier Park study area (Fig. 4) was divided into drainage or mountain complex units. Observation routes were established within each unit and systematic surveys made while covering the area by foot. All units were intensively surveyed at least once each month from May through September in 1974 and 1975, with more limited surveys performed in other months. During 1976, wintering herds were studied during March and April, and each survey unit was thoroughly covered at least once between June and September. As goat herds remained confined to wintering areas during May each year they were observed in the park, data from May are considered representative of winter conditions in Tables 1, 3, 4, 5, 6, 7, and 16. All park sightings were located with reference to 100-m Universal Transverse Mercator (UTM) coordinate intervals and plotted together on topographic maps (scale 1:24,000) after each monthly survey for additional analysis of spacing and range relationships. Habitat data recorded for each observation included zone (alpine or forest), type of vegetative community, slope, exposure (aspect), elevation, and presence or absence of shade. Goat locations were further described in terms of ledge development (a structural parameter used to evaluate availability of footing) and distribution along gradients of primary succession (soil development) and topographic moisture (water distribution and retention) (Kessell 1976). Twelve goats were temporarily marked with patches of natural-colored, unobtrusive dyes by projectile darts.
|
| Fig. 4. Location of summer and winter mountain goat ranges in the Glacier National Park study area. Numbers indicate average numbers of goats present on winter ranges during the 1974-76 study period. |
With the onset of parturition in May, I separated goats into 4 subadult classes (kid, yearling, 2-year-old male, and 2-year-old female), as well as adult male and adult female categories. Two-year-old females were considered nonparous and distinguished from adult females in sex and age ratios. As a check on field age and sex determinations, periodic trips were made to Sperry Chalet and Gunsight Pass in Glacier Park where goats were habituated to people and could be observed from as close as 2 m. Close-up observation and photography with high-speed film and telephoto lenses were used in an effort to develop an age structure for adult goats based upon horn annuli.
Types and intensities of agonistic social interactions within a fixed time interval were monitored, and motion picture photography was utilized to aid interpretation of behavioral sequences in both study areas
HABITAT RELATIONSHIPS
Habitat Selection
Studies of mountain goats show them to be intimately associated with high-elevation precipitous habitats in the earliest stages of primary succession, mainly rock outcroppings and fellfield slopes (Saunders 1955, Kuck 1970, Peck 1972, Smith 1976). Though subject to strong erosive forces, plant communities on ledges and fellfields tend to be stable and self-perpetuating, with changes in community composition occurring on a geologic time scale.
Goats consistently favored the newest geomorphic stages of available terrain in all months; therefore, use of precipitous areas was studied in relation to underlying rock strata in Glacier Park. Argillaceous rocks of the Appekunny, Grinnell, Shepard, and Kintla formations (Ross 1959) are loosely consolidated and erode to form layered ledge habitat with abundant step-like footing. More resistant Altyn and Siyeh limestone formations generally occur as massive blocks and present a more difficult terrain for goats to negotiate. Since ledge development is less marked within limestone strata, soil development and forage abundance are reduced. Winter ranges supporting the greatest densities of goats coincided with exposures of Appekunny and Grinnell argillites. Altyn limestone received little use, while Siyeh limestone, which overlies the Grinnell formation, was mainly associated with summer range.
Table 1 indicates that ledge habitats on rock outcroppings in Glacier Park were used by mountain goats for most feeding and nearly all bedding activities. Table 2 shows a similar pattern of preference for ledges by foraging mountain goats in the Swan Mountains (Chadwick 1974). More than 95 percent of goat bedsites in the Swan Mountains were on ledges during all months. Goats appeared to take greater care in choosing precipitous outcroppings for evening beds than for daybeds, probably to fulfill security requirements. Winter habitat selection in both areas appeared to reflect snow conditions which rendered foraging and travel extremely difficult except on outcroppings and windblown ridges. Summer habitat selection was more diverse and use of fellfields and meadows adjacent to cliffs increased in both study areas. In Glacier Park, habitat selection trends over summer were shown to be associated with use of sites with better soil development (Table 3) and water retention qualities (Table 4). Use of forested habitats was rare in both study areas, despite the proximity of most winter ranges to timber stands. Infrequent use of forests by mountain goats elsewhere in Montana has been noted by Saunders (1955), Peck (1972), and Smith (1976).
Table 1. Percent utilization of vegetation communities by mountain goats in Glacier National Park study area, 1974-76. (N=1,519)
| Vegetation community | May |
June |
July |
August |
September | |||||
| Feeding | Bedding | Feeding | Bedding | Feeding | Bedding | Feeding | Bedding | Feeding | Bedding | |
| Permanent snow/icefield | 1 | 0 | 0 | 0 | 1 | 4 | 1 | 2 | 0 | 0 |
| Dry ledge | 68 | 74 | 61 | 58 | 50 | 51 | 29 | 52 | 24 | 48 |
| Wet ledge | 20 | 23 | 16 | 15 | 14 | 16 | 12 | 16 | 17 | 28 |
| Talus/scree/moraine | 4 | 1 | 6 | 3 | 18 | 13 | 13 | 10 | 12 | 8 |
| Bunchgrass meadow/tundra | 4 | 2 | 10 | 14 | 10 | 13 | 28 | 14 | 18 | 8 |
| Wet meadow | 1 | 0 | 0 | 0 | 2 | 3 | 8 | 2 | 3 | 0 |
| Shrubfield/ravine | 2 | 0 | 6 | 10 | 5 | 0 | 9 | 4 | 26 | 8 |
| Forest | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 2. Percent utilization of vegetation communities by foraging mountain goats in the Swan Mountains study area, 1971-74. (N=4,570)
| Vegetation community | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec |
| Cliff ledge | 53 | 26 | 25 | 88 | 63 | 56 | 36 | 26 | 42 | 16 | 49 | 85 |
| Ridgetop ledge | 0 | 74 | 75 | 0 | 3 | 11 | 3 | 2 | 4 | 2 | 10 | 15 |
| Ravine/wet meadow | 0 | 0 | 0 | 5 | 5 | 9 | 26 | 31 | 18 | 11 | 23 | 0 |
| Dry meadow | 0 | 0 | 0 | 5 | 27 | 22 | 33 | 37 | 36 | 71 | 18 | 0 |
| Forest | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 4 | 0 | 0 | 0 | 0 |
Table 3. Percent habitat utilization along a soil succession gradient by mountain goats in Glacier National Park study area, 1974-76. (N=1,519)
| Primary succession stage | May |
June |
July |
August |
September | |||||
| Feeding | Bedding | Feeding | Bedding | Feeding | Bedding | Feeding | Bedding | Feeding | Bedding | |
| Glacier/icefield | 0 | 0 | 0 | 0 | 1 | 4 | 2 | 2 | 0 | 0 |
| Solid outcrop | 62 | 67 | 47 | 48 | 35 | 31 | 24 | 44 | 26 | 48 |
| Broken outcrop | 33 | 32 | 14 | 21 | 23 | 27 | 15 | 19 | 14 | 28 |
| Boulders and talus | 1 | 1 | 3 | 0 | 9 | 12 | 4 | 9 | 8 | 5 |
| Talus | 1 | 0 | 7 | 0 | 3 | 7 | 6 | 1 | 3 | 3 |
| Talus and meadow | 1 | 0 | 9 | 10 | 17 | 7 | 21 | 12 | 15 | 6 |
| Meadow | 1 | 0 | 1 | 4 | 8 | 12 | 8 | 2 | 9 | 8 |
| Meadow and shub krummholz | 1 | 0 | 1 | 17 | 3 | 0 | 18 | 11 | 18 | 2 |
| Shrub/krummholz | 0 | 0 | 6 | 0 | 1 | 0 | 1 | 0 | 5 | 0 |
| Shrub/krummholz and forest | 0 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 2 | 0 |
| Typical forest | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Table 4. Percent habitat utilization along a topographic moisture gradient by mountain goats in Glacier National Park study area, 1974-76. (N=1,519)
| Topography | May | June | July | August | September | |
| (Wet) | Hydric basin | 0 | 0 | 0 | 2 | 0 |
 |
Ravine | 2 | 1 | 0 | 3 | 1 |
| Draw | 0 | 1 | 1 | 4 | 6 | |
| Sheltered slope | 19 | 18 | 17 | 20 | 34 | |
| Open slope | 76 | 74 | 30 | 58 | 54 | |
| Peaks and ridges | 3 | 6 | 50 | 11 | 5 | |
| (Dry) | Xeric flats | 0 | 0 | 2 | 2 | 0 |
More than two-thirds of the slopes used by mountain goats in Glacier Park exceeded 400 (Table 5). Average steepness of slope used was found to increase under winter conditions in both study areas. This largely reflected confinement of goats to those cliff areas with the greatest snow-shedding qualities; at comparable aspects and elevations, steep cliff terrain retains less snow than more moderate slopes. Kuck (1970) found over 60 percent of all wintering goats on slopes steeper than 50deg.
Table 5. Percent utilization of slopes by mountain goats while feeding and bedding, Glacier National Park study area, 1974-76. Slopes are presented as degrees from horizontal. (N=1,519)
| Month | Feeding |
Bedding | ||||
| 0—40° | 41—60° | 61—90° | 0—40° | 41—60° | 61—90° | |
| May | 31 | 53 | 16 | 30 | 50 | 20 |
| June | 13 | 71 | 16 | 19 | 77 | 4 |
| July | 33 | 59 | 8 | 40 | 57 | 3 |
| August | 41 | 53 | 6 | 24 | 62 | 14 |
| September | 51 | 46 | 3 | 40 | 57 | 3 |
| Average, all groups | 34 | 56 | 10 | 30 | 61 | 9 |
| Average, groups larger than 5 (N=135) | 66 | 31 | 3 | 45 | 42 | 13 |
Ninety-eight percent of all goat sightings in Glacier Park were above 1,220 m elevation (Table 6). After May, goats increased their use of high elevations and decreased their use of southerly aspects (Table 7). Northerly aspects provided both shade and moist micro-climates during hot weather. Peck (1972) noted that shaded sites were favored by goats for most summer activities and that northern aspects received more summer use than other aspects in the Spanish Peaks, Montana.
Table 6. Percent utilization of different elevations by mountain goats, Glacier National Park study area, 1974-76. (N= 1,519)
| Elevation (m) | May | June | July | August | September |
| 945—1,220 | 2 | 0 | 0 | 0 | 0 |
| 1,250—1,525 | 22 | 2 | 1 | 0 | 1 |
| 1,555—1,830 | 43 | 17 | 3 | 4 | 16 |
| 1,860—2,135 | 31 | 54 | 30 | 38 | 38 |
| 2,165—2,440 | 2 | 25 | 52 | 50 | 42 |
| 2,470—2,755 | 0 | 2 | 14 | 8 | 3 |
Table 7. Percent utilization of different aspects by mountain goats, Glacier National Park study area, 1974-76. (N=1,519)
| Aspect | May | June | July | August | September |
| North | 5 | 17 | 18 | 18 | 11 |
| East | 26 | 36 | 37 | 32 | 28 |
| South | 60 | 32 | 22 | 28 | 41 |
| West | 9 | 15 | 23 | 22 | 20 |
Mountain goat populations were found to summer and winter at consistently higher elevations and use ledge habitat and steep slopes to a far greater extent than any other large mammal in the ecosystems studied. In Glacier Park, less than 1 percent of all goat sightings were within 1 km of a cervid observed during the same survey. Five to 10 percent of the largely subalpine winter goat range in the Swan Mountains overlapped that of cervids. Singer (1975) used nomograms to effectively depict the extent of mountain goat niche isolation relative to wintering cervids in southern Glacier Park. Holyrod (1967) stated that goats were isolated from all other ungulates in the Mt. Wardle study area in Kootenay National Park, British Columbia. About 10 percent of the Glacier Park study population shared moderate slopes and lower-elevation terrain with bighorn sheep, mostly on the east side of the Continental Divide in the St. Mary and Swiftcurrent drainages. Range overlap was most noticeable from late spring through fall. The two species were observed bedded and feeding within several meters of one another. Interspecific dominance was not readily apparent. Females of both species exhibited signs of anxiety in the presence of males of the other species, and each species appeared to initiate avoidance movements when confronted by greater numbers of individuals of the other species. Bighorn rams were twice seen directing courtship behavior toward female goats. Overt competition for resources appeared minimal. R. Riggs (personal communication) noted goats and bighorn sheep in the same vicinity only 4 times during the winter of 1975-76 while investigating bighorn sheep winter ecology in the study area. Geist (1971) noted no serious interspecific competition for winter forage in his observations of these sympatric bovids in the Cassiar Range of British Columbia.
Food Habits
Anderson (1940), Casebeer (1948), Holroyd (1967), Kuck (1970), and Peck (1972) reported shrubby browse to be the staple winter food of goats, while Brandborg (1955), Saunders (1955), and Hibbs (1967) noted dominant winter use of grasses and sedges. Heavy winter utilization of conifers (Geist 1962), ferns (Hjeljord 1973), and lichens (Hanson 1950, Brandborg 1955) further emphasizes dietary flexibility and suggests that mountain goat food habits are readily adaptable to availability of forage classes.
Data from the Swan Mountains point out dietary variation within as well as between populations. Herds on the eastern slope of the Swan Mountains relied upon grasses and sedges for the bulk of their winter diet, while browse items constituted only 11.5 percent of winter food (Chadwick 1974). However, on west-slope wintering areas, 10-20 km distant, mountain goats, some of which were in occasional contact with east-slope herds, consumed approximately equal amounts of browse and grasses. Utilization patterns appeared clearly related to availability since west-slope cliffs were lower in elevation and supported substantially more browse than outcroppings on eastern slopes. Unquantified observations revealed a similar situation in Glacier Park: extensive browsing on west-slope ranges and almost exclusive grass and sedge use on eastern ranges, again consistent with the relative availabilities of these forage classes.
Plasticity of diet and feeding style are essential for goats to exploit microniche resources in winter, when forage and mobility are limited. Goats made use of their excellent climbing abilities to reach the outermost edges of cliff ledges where snowdepths were usually reduced by winds and snow slippage. They also exploited areas such as the bases of fallen logs and boulders where absorption and reflection of thermal energy and reduced snowfall made more for age available. Goats were able to reach sheltered crannies and fissures on steep rock walls where plants were exposed and snow did not accumulate. Forage cover on such vertical substrate was measured and found to be as high as 50 percent on many sites, composed chiefly of nonvascular plants such as edible lichen, mosses, and clubmosses, as well as ferns and a few evergreen forbs. In addition to their availability, such, microniche plant species may be of particular nutritional importance. The value of green forage intake during winter has been discussed by Karaer (1961) for reindeer (Rangifer tarandus), Hoefs (1974) for Dall sheep (Ovis dalli dalli), and Hjeljord (1973) for mountain goats.
Goats are able to contend with deep-snow conditions through the aid of highly developed pawing abilities. Geist (1971) found mountain goat pawing frequencies to be higher than those of bighorn sheep. Nevertheless, heavy, prolonged snows, or rains followed by frosts which formed thick, icy crusts often prompted alternate feeding strategies. Severe snowstorms or crusts stimulated movement of goats from lower cliffs to higher, windswept outcroppings and ridgetops on some ranges in both study areas. Alternately, goats moved downward or laterally, away from optimum cliff terrain, toward slopes supporting shrubfields. The latter option was more common in late winter when packing and settling permitted goats to travel over deep snow accumulations without sinking so deeply that their efforts were unrewarding. Hjeljord (1973) observed similar shifts from hillside to ridgetop in response to winter forage availability, and Holroyd (1967) found goats shifted to exclusive use of browse under crusted snow conditions.
It should be noted that young goats were most often at a disadvantage in pawing through deep snows and crusts to obtain buried forage. As a result, they often fed in craters pawed and utilized by older animals, and they traveled in paths of other goats. Kids were especially reliant upon their mothers for such opportunities. Kids were also able to bed in deep craters pawed by their mothers for protection from winds typically lying in contact with the leeward or uphill side of her body. Kids and yearlings were frequently observed to continue feeding after older goats had bedded following a feeding session. This suggests that foraging efficiency relative to metabolic requirements was lower in juvenile classes.
Summer food habits reflected increased mobility of goats and a pronounced preference for succulent plant tissues in early phenological stages. South-facing winter range cliff outcroppings were generally the first habitats to support green spring forage. From these, goats typically moved upward in elevation, exploiting new plant growth, especially inflorescences, of a succession of species until cliff and ridgetop ledge vegetation dried. Thereafter, goats moved slightly downward, seeking succulent forage in moist meadows, basins, and ravines. Klein (1970) pointed out the value to herbivores in arctic and alpine regions of being able to range over a wide array of elevations and exposures to exploit early physiological stages of plant growth for as long a period as possible during the short growing season. Other studies have demonstrated the nutritional superiority of early growth stages (Oelberg 1956) and of high-elevation forage over that of lower elevations (Johnston et al. 1968). Goats in the Swan Range have been recorded using 168 different plant species to date, most of them recently flowering forbs.
Range
General Considerations
Available evidence strongly suggests that traditional use of specific ranges is characteristic of the species. Brandborg (1955) recorded a known adult female on the same winter range for 3 consecutive years. Smith (1976) noted home range fidelity over a period of 3-4 years for 4 marked individuals (3 females, 1 male) in the Bitterroot Mountains, Montana. Four marked females were observed on the same summer and winter range in the Swan Mountains during 4 consecutive years. Naturally identifiable individuals provided examples of winter range fidelity in Glacier Park over a 3-year period. Circumstantial evidence from unmarked goats also reinforced available data insofar as similar groups were observed in the same locations each year. Continuous observation of a single herd in the Swan Mountains revealed a constant female and subadult composition for up to 18 months. As another example, a group of 3-4 large males, 1 of which possessed unusually long horns, was recorded during July each year from 1974 through 1976 on the same talus slope in the vicinity of Ahern Pass in Glacier Park, some distance from any other male group.
Use patterns within home ranges were also quite constant. Daily charting of individual goat activities in the Swan Mountains showed that use of trails, specific feeding sites, dustbathing areas, day-beds, evening beds, and escape routes were highly predictable. Individuals appeared to favor particular portions of available range and exhibited unique use patterns therein. The degree of specific site orientation by this species was further attested to by trails worn into rock substrata, dustbathing sites and bedding areas devoid of vegetation, and caves and crevices littered with deep pellet accumulations. Smith (1976) also documented traditional use of small "key" areas by individual goats. On winter ranges, vegetation in key areas may be heavily used while adjacent sites are subject to only light to insignificant use.
Habitual use of ranges and key areas within ranges is probably based upon learned traditions. Adult females remain on winter ranges, which are typically steeper and more rugged than transitional or summer ranges, to give birth in May or June. Isolated with its mother for a week or more, a neonate becomes familiar with the area immediately surrounding its birthsite during the postpartum period (Chadwick 1974). Kids are still closely following their nannies in the fall when the herd returns to winter range. Yearlings, dissociated from their mothers, follow other females during their second departure from and return to winter range. Females continue to follow other females in subsequent years.
Beginning as yearlings, males exhibit more wide-ranging, exploratory movement patterns than females. By 2 years of age, males begin to increase the size of their home range relative to that of females. Many depart from female-subadult areas in the process, as was observed with marked males in the Swan Mountains, and by 3 years of age usually occupy independent ranges, often in association with other males. Although males also evinced habitual use of ranges once established, they remained more likely than females to show variable movement patterns and to travel through atypical terrain.
All breeding took place on female-subadult wintering areas. Geist (1964) suggested and Chadwick (1974) and Kuck (personal communication, cited in Smith 1976) verified with marked animals that whereas females return to the same wintering areas each fall, rutting males cross between ranges. Adult males then spend at least early winter on whichever female range they occupy at the termination of the rut before returning to separate or peripheral range, usually by mid-winter, depending on snowdepths.
Glacier Park
Goat sightings during March, April, and May were used to illustrate winter-early spring range (Fig. 4). Continued presence of deep snow restricted goats to these relatively small, isolated ranges from October through May or early June. Ten major wintering areas were recognized; all were at elevations of 1,070-1,980 m on generally southern exposures. Areas occupied predominantly by males were found to be peripheral to or separate from female-subadult wintering concentrations.
During June, herds on both sides of the Continental Divide dispersed toward ridgetops in the wake of receding snows. The general pattern of transitional movements from winter to summer range was inferred from successive goat sighting locations, which were similar each year. Adult males were the first to leave wintering areas, and they remained the most mobile and widely dispersed population segment. Goat sightings during July and August were used to describe summer range. Winter ranges were entirely separate from one another, and less than 5 percent of summer range overlapped wintering areas. It is evident that summer range was more or less continuous and conformed to ridgeline topography. Because vertical migration patterns culminated in extensive summer utilization of peaks and ridgetops, goat population units acquired potential for exchange of members. This would be adaptive from the standpoint of genetic interchange, dispersal from high-density areas and restocking of low-density ranges, and transfer of learned information regarding salt sources and key or alternate forage supplies. Actual magnitude of population interchange is unknown, but there was evidence of at least temporary mixing of herds during summer in several portions of the study area. Singer (1975) found goats from widely separate ranges traveling at least 15 km, some of them traveling through forested habitats or swimming a river en route, to use the Walton Lick in southern Glacier Park.
Interspecific behavior involving goats seeking salt from humans has been investigated in Glacier Park by Bansner (1976). The spread of specific behavior patterns from the Sperry Chalet-Gunsight Pass area to Hidden Lake and the Highline Trail in the study area apparently involves communication of these learned salt-specific activities between herds (Chadwick 1977).
During September and October, goats converged toward wintering areas in a reversal of spring transition movements. Early snowstorms prompted temporary fall use of winter range, followed by return to higher elevations if snows melted.
Swan Mountains
Cliff habitat in the Swan Mountains is discontinuous and surrounded by extensive forested terrain with little precipitous escape cover. Consequently, both winter and summer ranges were small in comparison with those of Glacier Park (Fig. 3). Marked females remained in the same winter-summer ranges of no more than 15-25 km2 over a 4-year period, winter range lying almost entirely within summer range. Data from marked and recognizable individuals suggested that female-subadult herds were relatively isolated from one another throughout the year. As in Glacier Park, males occupied separate or peripheral ranges. Males were found to travel between female ranges and were the main source of contact between population units.
Existing range use patterns in the Swan Mountains are difficult to interpret as they may be influenced by various human disturbances documented by Chadwick (1974). According to many hunters and long time residents of the area, goats were previously more abundant and occupied an area larger than that presently used (Fig. 3). This is supported by mountain goat harvest records for the Swan Mountains (Chadwick 1974), which show substantial declines in hunting districts 130 and 140, which included the west and east sides of the Swan Mountains, respectively. Range use investigations on 6 west-slope wintering areas revealed that less than 5 percent of available favored grass species and less than 10 percent of available favored browse species were consumed over winter on key use sites. This also suggests understocking.
POPULATION CHARACTERISTICS
Productivity
Table 8 presents population structure for the Glacier Park study area from 1974 through 1976. Kidding season extended from 20 May through 15 June, though births were concentrated in the last week of May and the first week of June. Exceptions did occur, suggesting recurrent estrus or departure from the normal gestation period of approximately 178 days first described by Seton (1927). Casebeer et al. (1950) cited a record of newborn goats observed on 27 February 1937, and J. G. Edwards reported an adult female with a newborn kid on 28 August 1975 in Glacier Park. Edwards observed the female ingesting the afterbirth at this time, indicating that parturition had occurred within several hours (Chadwick 1974). I subsequently observed a neonate in the same vicinity of Mt. Wilbur on 2 occasions. Physical and behavioral traits indicated that the kid, probably the same one as seen by Edwards, was less than 2 weeks old in early September.
Table 8. Mountain goat population structure, Glacier National Park. Data are presented as number per 100 adult females / percent in total population. Proportion of males observed in surveys was variable as the largely solitary males dispersed early from winter ranges and became difficult to locate during summer.
| Class | Study area |
Bansner 1974 |
Singer 1975 |
Petrides 1948 | ||
| 1974 | 1975 | 1976 | ||||
| Kid | 56/18 | 55/19 | 57/20 | 39/ | 75/ | /42 |
| Yearling | 36/12 | 33/12 | 40/14 | 6/ | 33/ | |
| Two-year-old | 36/12 | 20/7 | 28/10 | 41/ | 21/ | |
| Adult male | 86/27 | 75/26 | 57/20 | 47/ | 52/ | |
| Adult female | 100/32 | 100/35 | 100/35 | 100/ | 100/ | |
| Unclassified juvenile | 12/ | 5/ | ||||
| Unclassified adult | 14/ | 26/ | ||||
| Unclassified older than kid | /100 | |||||
| Number classifieda | 314 | 300 | 232 | 171 | 745 | 132 |
aStudy area figures are based upon complete, unduplicated counts, while those of Bansner (1974), Singer (1975), and Petrides (1948) lump all goat observations, including resightings. | ||||||
Natality was nearly identical each year in the Glacier Park study area, averaging 57 kids/100 adult females. Twins accounted for 7-11 percent of the annual recruitment of young in 1974, 14-17 percent in 1975, and 4-7 percent in 1976: an average of 10 percent. Continuous observation is necessary to distinguish twins from playmates. Two, 3, and even 4 unrelated kids were observed following a single adult female for over 20 minutes before returning to their mothers.
There is no evidence that females breed as yearlings even under conditions stimulating unusually high natality such as introduction to favorable new ranges (Lentfer 1955, Moorhead personal communication).
Juvenile mortality is fairly high in the species, and occurs mainly during the late winter-early spring period (Casebeer et al. 1950, Brandborg 1955, Smith 1976). From 1974 to 1975, in which moderately severe winter conditions prevailed, 41 percent of the kid cohort and 44 percent of the yearling cohort were lost. From 1975 to 1976, under a light winter regime, 27 percent of the kid cohort and 15 percent of the yearling cohort disappeared. The unusually high proportion of 2-year-olds in the 1974 study area population and in populations using the Walton Lick that year (Bansner 1974) probably reflect very low mortality in the kid class over the unusually light winter of 1972-73.
Although natality was similar east and west of the divide in Glacier Park, juvenile survival was consistently higher on west-slope ranges (Table 9). Sex did not appear to influence subadult survival as is evident from approximately equal percentages of 2-year-old males and females. Slightly fewer adult males were present than adult females, a condition found in nearly all studies of this species. Some of the disparity in male:female counts is probably an artifact of survey limitations. Adult males are more solitary, disperse earlier and more widely from wintering sites, and favor more rugged terrain than other classes, all of which makes them more difficult to locate. The low male:female ratio obtained in 1976 is primarily a result of the fact that surveys were made less often and later in summer than in 1974 or 1975.
Table 9. Comparison of subadult population structures east and west of the Continental Divide, Glacier National Park study area, 1974-76. Data are presented as number per 100 adult females.
| Year | N | Kid | Yearling | Two-year-old | ||
| Total | Male | Female | ||||
| West side | ||||||
| 1974 | 47 | 58.9 | 60.7 | 39.3 | 10.7 | 28.6 |
| 1975 | 44 | 69.2 | 56.0 | 40.0 | 24.0 | 16.0 |
| 1976 | 36 | 50.0 | 68.2 | 45.5 | 27.3 | 18.2 |
| East side | ||||||
| 1974 | 84 | 62.1 | 29.7 | 34.1 | 13.8 | 20.3 |
| 1975 | 69 | 50.0 | 38.9 | 13.0 | 5.6 | 7.4 |
| 1976 | 67 | 59.0 | 29.5 | 21.3 | 11.5 | 9.8 |
Population structure in the Swan Mountains study area may have been influenced by various artificial factors (Chadwick 1974). Sex and age ratios relative to 100 adult females were artificially high due to removal by hunters of more adult females than other classes during recent years. Sample sizes are too small to permit intensive analysis, although the 1973 census, conducted by 5 investigators throughout the summer and fall, is thought to include almost every goat inhabiting the study area. Both productivity and subadult survival were highest following the light winter of 1972-73.
Mortality
Table 10 provides ages of a sample population of 52 adult mountain goats in Glacier Park based upon field counts of horn annuli. Field aging of adults, even when aided by photography, was difficult. Basal annuli tended to be indistinct and partially covered by hair, especially in older animals, thereby increasing the likelihood of age underestimation. Further testing and refinement of this technique is necessary, but it is presently felt that individual ages were under estimated by at most 2 years. This was compensated for in Table 12 by adding 1 year to the age of animals whose annuli count was judged to be of "good" instead of "excellent" quality, and 2 years to annuli counts judged to be of "fair" quality. Counts of less than "fair" quality were discarded. Animals over 8 years of age comprised 11.5 percent of the sample. Table 10 also shows horn annuli counts from 8 adult carcasses. The oldest age recorded for a living goat was 13, and for a carcass, 12. Brandborg (1955) noted extreme wear and frequent loss of teeth in mountain goats older than 8. Records from hunting harvests and taxidermists suggest that longevity beyond 12 or 13 years is rare.
Table 10. Age distribution of adult goats as estimated from horn annuli counts of study area sample population and carcasses, Glacier National Park. To compensate for underestimation resulting from indistinct or hair-covered annuli, ages were adjusted by adding 1 year to age estimates considered to be "good," and 2 years to age estimated considered to be "fair." All counts of less than fair quality were discarded.
| Sex | Adjusted age (years) |
Total | ||||||
| 3 | 4 | 5 | 6 | 7 | 8 | 8+ | ||
| Sample population | ||||||||
| Male | 4 | 3 | 6 | 1 | 0 | 2 | 1 | 17 |
| Female | 11 | 5 | 2 | 6 | 3 | 3 | 5 | 35 |
| Both sexes | 15 | 8 | 8 | 7 | 3 | 5 | 6 | 52 |
| Carcasses | ||||||||
| Male | 1 | 0 | 0 | 0 | 1 | 1 | 1 | 4 |
| Female | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 4 |
| Both sexes | 2 | 0 | 2 | 0 | 1 | 1 | 2 | 8 |
| Total sample | ||||||||
| Male | 5 | 3 | 6 | 1 | 1 | 3 | 2 | 21 |
| Female | 12 | 5 | 4 | 6 | 3 | 3 | 6 | 39 |
| Both sexes | 17 | 8 | 10 | 7 | 4 | 6 | 8 | 60 |
Apart from senility, sources of mortality to mountain goats are poorly understood. As a consequence of its habitat specialization, the goat must deal with what mountaineers refer to as "objective danger": certain inevitable and unpredictable physical hazards associated with steep slopes, such as rock- and icefalls, and avalanches. In a lifetime of climbing, goats must also contend with the possibility of snow cornices (on which they often travel) or rock ledges giving way beneath their weight. Finally, there is the constant danger of simply misplacing a foot and losing balance, not only under typical climbing conditions, but under unusually wet or icy circumstances, or when avoiding predators or aggressive conspecifics. Brandborg (1955), Holroyd (1967), and Hjeljord (1971) discussed the possibly major role of avalanches and climbing accidents in goat mortality.
Eighteen carcasses were discovered within the Glacier Park study area. Ten additional carcasses were located by myself and other park personnel in adjacent park locations. In 20 cases where probable cause of death could be determined, 12 (60 percent) were attributed to avalanches and 3 (15 percent) to climbing accidents. Two were killed by mountain lions at an artificial salt source near Sperry Chalet, 2 were killed by grizzly bears, and 1 apparently starved during a severe winter storm. With the exception of 1 kid, all carcasses were judged to be 2 years old or older on the basis of horn annuli, dentition, bone length, and pelage characteristics. All but 1 of 22 carcasses found in previous years by park personnel were reportedly located in snowslides. Natural mortality of goats in southern Glacier Park and adjacent National Forest lands as reported by Singer (1975) was entirely due to avalanches. Only 1 carcass other than those taken by hunters was located in the Swan Mountains, where it was discovered in a snowslide.
Evidence of injury, disease, and other physical abnormalities in live goats in both study areas is presented in Table 11. Despite occasionally heavy infestations of ticks (Dermacentor andersoni) serious debilitation from parasites or disease of any sort was not detected, nor has any present or historical evidence of epizootic conditions among goats in either area been recorded. Most common were horn breakage and deformity and foot injuries, all of which may be indicative of climbing mishaps. In 4,400 goat-hours (number of goats X number of hours observed) in the Swan Mountains, I witnessed 29 missteps leading to loss of balance (Table 12), a rate of 1 misstep every 151.7 goat-hours. Actual rates were higher, as Table 12 does not include kids less than 3 months of age, which frequently lost their footing, and it does not include those scrambling, leaping maneuvers resulting from rapid climbing assaults, since these were difficult to interpret. The majority of mishaps recorded (17) occurred on wet or icy terrain. Older goats were generally methodical climbers in comparison with subadults. Kids and yearlings often negotiated areas in an all-or-nothing fashion and provided most of the frantic mid-air reversals and leaps to safety witnessed but not included in Table 12. It was also my impression that younger goats, through experimentation or inexperience and lack of familiarity with the terrain, most frequently placed themselves in difficult mountaineering situations. Kids and yearlings also experienced the greatest difficulty with crusted and granular snow on steep terrain as they lacked sufficient weight to penetrate it and secure footholds.
Table 11. Evidence of disease, injury, and physical abnormalities among mountain goats in the Glacier National Park study area, Glacier Park Sperry-Gunsight area, and Swan Mountains study area. High incidence of disease and injury in the Sperry-Gunsight area (annual population, about 60) compared to Glacier Park and Swan Mountains study areas (annual populations, 360 and 80, respectively) may reflect closer observation and/or the attraction of afflicted individuals to salt sources in the Sperry-Gunsight area. Sperry-Gunsight data are primarily from Bansner (personal communication).
| Type of evidence | Number of goats affected |
Remarks | ||
| Glacier Park study area |
Glacier Park, Sperry-Gunsight area |
Swan Mountains study area | ||
| Horns bent, broken, or missing | 11 | 6 | 5 | |
| Foot or leg injuries causing limp | 6 | 6 | 5 | |
| Diarrhea | 4 | 2 | 0 | Observed primarily during August and September. |
| Abnormal pelage | 3 | 1 | 1 | Coat extremely irregular, rough, and mottled. Possibly affected by mites. |
| Submaxillary swelling | 0 | 5 | 0 | Possible necrotic stomatitis. |
| Possible puncture wound | 0 | 2 | 3 | One male missing an eye. |
| Possible respiratory difficulties | 0 | 0 | 1 | Periodic coughing combined with long periods of inactivity. |
Table 12. Potentially harmful climbing events recorded during 4,400 goat-hours of observation of mountain goats in the Swan Mountains. Data are presented as absolute number/adjusted number based on number in class / 100 adult females. Aggression-related events resulted from 192 agonistic encounters in dangerously steep terrain. (Modified from Chadwick 1974)
| Type of event | Kid | Yearling | Two-year-old |
Adult |
Unclassified | Total | ||||
| Male | Female | Male | Female | |||||||
| Knocked over edge by hard blow | 0 | 2/5.0 | 1/3.3 | 0 | 0 | 0 | 0 | 3 | ||
| Pushed or prodded over edge | 7/10.1 | 7/17.5 | 0 | 0 | 0 | 1/ | 0 | 15 | ||
| Forced to leap over edge to avoid aggressor | 10/14.4 | 5/12.5 | 1/3.3 | 0 | 0 | 2/ | 0 | 18 | ||
| Leaped or knocked over edge as result of another encounter (innocent bystander) | 0 | 0 | 0 | 1/3.3 | 0 | 1/ | 0 | 2 | ||
| Aggressor loses footing during pursuit | 0 | 0 | 0 | 0 | 0 | 1/ | 0 | 1 | ||
| Total aggression-related events | 17/24.5 | 14/35.0 | 2/6.7 | 1/3.3 | 0 | 5/ | 0 | 39 | ||
| Climbing misstep leading to loss of balance | 5/7.2 | 5/12.5 | 3/10.0 | 0 | 0 | 9/ | 7/ | 29 | ||
| Total of all events | 22/31.7 | 19/47.5 | 5/16.7 | 1/3.3 | 0 | 14/ | 7/ | 68 | ||
In addition to missteps which occurred under ordinary climbing conditions, 291 agonistic social interactions took place on steep terrain during the same 4,400 goat-hours. Thirty-nine (13.4 percent) of these resulted in potentially harmful climbing events which primarily affected subadults (Table 11). This yields a rate of 1 climbing mishap due to aggression every 112.8 goat-hours, higher than that recorded for normal climbing activities. Smith (1976) described an incident of an older goat butting a kid from a ledge, causing it to fall some distance.
Table 13 summarizes mountain goat-predator interactions. Although mountain goats were the most abundant ungulate in the Glacier Park study area in terms of numbers and biomass, evidence of successful predation was rare. None was discovered in the Swan Mountains, nor has significant predator pressure been reported by other investigators. This is probably due to 2 factors: (1) habitat specialization which reduces potential predator-goat contact and provides dense security cover in a topographic context; and (2) the ability of goats older than 1 year to inflict lethal wounds with their horns. Aggressive defense of young by mothers was observed, and adult goats were seen to intimidate lynx, wolverine, and coyotes. Trapped goats displayed aggressive resistance when we attempted to immobilize them, repeatedly charging and making horn thrusts. At least 4 people have been butted by aggressive salt-seeking goats in the Sperry Chalet-Gunsight Pass area (Bansner 1976). It is unlikely that goats in either study area constituted an important dietary item for any predator species except possibly as carrion. Grizzly bears regularly searched the bases of cliffs below winter ranges for carcasses during late April and May.
Table 13. Mountain goat-predator relationships in the Glacier National Park study area, 1974-75. Simultaneous observation of mountain goats and a predator less than 1 km apart was recorded as an interaction, even if one or both species appeared unaware of the other's presence. Due to the frequency with which eagles were observed in mountain goat range, goat-eagle interactions were recorded only when eagles passed within 200 m of goats.
| Predator | Number of interactions |
Number of different predators |
Number of goats involved |
Average interspecific distance (m) |
Remarks |
| Eagle | 16 | 15 | 77 | 68 | Some harassment observed. No direct attacks. |
| Lynx | 1 | 1 | 1 | 5 | Lynx approached and circled 3-year-old male. No attack. |
| Wolverine | 18 | 2 | 45 | 379 | No attacks. Closest approach within 5 m. |
| Grizzly bear | 9 | 6 | 30 | 433 | Includes 2 unsuccessful attacks, 1 kill, 1 probable kill. |
| Total | 44 | 24 | 153 | ||
A possible source of mortality to kids is separation from their mother prior to 10-11 months of age. Kids tended to follow the closest large goat during sudden flight and occasionally became separated from their mothers. Separation also occurred during normal activities and, of course, in the event of the nanny's death. Twenty-two instances of separation were recorded, 12 of them permanent.
Most orphaned kids attempted to follow other adult females closely, but females were typically intolerant of alien kids and behaved aggressively toward them. Mountain goat mother-infant bonds are established during the early postpartum period through frequent olfactory contact (Chadwick 1974). When confusion of identity arises, as in the case of mothers seeking to separate playing youngsters, perianal odors are used to distinguish offspring from other kids. No nursing, acceptance within critical personal space, or other maternal behavior was directed toward alien kids by adult females, although temporary adoption of yearlings and 2-year-olds by females without kids was observed by Bansner (personal communication) on 2 occasions and myself on 8 occasions. Four of these adopted subadults were observed nursing lactating females during summer, implying probable recent loss of young by the females and redirection of maternal behavior toward subadults. It is possible that these subadults were offspring of previous years and that olfactory recognition was essential to acceptance. If this were not the case, then orphaned kids might be able to attach themselves to such females if circumstances permit association in a group. In the absence of maternal protection, orphaned kids were the least dominant members of all groups and suffered harassment by older subadults, notably yearlings. Probably as a consequence of unsatisfactory social status, orphans were seen on at least 3 occasions to cease following bands and remain solitary in a small home range for up to several weeks. Although 2 orphaned kids survived the unusually light winter of 1972-73 in apparently good condition, orphans would probably be at considerable disadvantage in surviving moderate to severe winter conditions.
Injury and mortality to males from fighting during the rut has been reported by Seton (1927), Geist (1964, 1967), and deBock (1970). DeBock concluded that early winter conditions of deep snow can restrict mobility and intensify competition for females in an area, thereby increasing the frequency of male combat and resulting injuries. Table 13 shows that 5 goats in the study areas bore possible puncture wounds, but no major injuries were inflicted during those battles observed between males or between any other classes.
Grouping
Group Size
Mountain goats further than 50-75 m apart were considered separate if they showed no obvious signs of related activity or orientation toward one another. Mean group size varied between 1.9 and 3.5 in Glacier Park and 2.1 and 4.6 in the Swan Mountains (Table 14). Aggregate indices (Jarman 1973) did not exceed 7 in either population during any month. Fifty-three percent of all park goat groups sighted were solitary animals (group size=1), and an additional 25 percent of groups were pairs. Expressed as percent of the total number of goats sighted, 35 percent of the park population occurred alone or with 1 other goat. A high percentage of these pairs were females with young, behaviorally equivalent to solitary animals. A picture therefore emerges of the mountain goat as more of a semi-gregarious animal than a typical herd species.
Table 14. Mean group size and aggregate index (Jarman 1973) of mountain goats in the Glacier National Park and Swan Mountains study areas. Swan Mountains data are for 1971-73 combined.
| N | Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | Annual average | |
| Glacier Park | ||||||||||||||
| Mean | ||||||||||||||
| 1974 | 626 | — | — | — | — | 1.9 | 2.1 | 2.4 | 2.9 | 3.3 | — | — | — | 2.5 |
| 1975 | 492 | — | — | — | — | 1.8 | 3.0 | 3.2 | 3.5 | 3.3 | — | — | — | 3.0 |
| Aggregate index | ||||||||||||||
| 1974 | 626 | — | — | — | — | 3.3 | 4.1 | 6.8 | 7.1 | 8.3 | — | — | — | 5.9 |
| 1975 | 492 | — | — | — | — | 3.0 | 6.8 | 7.7 | 7.5 | 7.8 | — | — | — | 6.6 |
| Swan Mountains | ||||||||||||||
| Mean | 4,570 | 3.1 | 3.3 | 3.6 | 2.8 | 2.1 | 4.5 | 4.6 | 2.9 | 2.6 | 3.6 | 2.4 | 2.6 | 3.2 |
| Aggregate index | 4,570 | 4.2 | 6.0 | 6.2 | 4.4 | 3.2 | 5.5 | 5.0 | 5.0 | 3.9 | 5.9 | 3.2 | 3.3 | 4.6 |
Annual mean group size from 1971 through 1973 averaged 3.2 in the Swan Mountains, and was 2.5 and 3.0 in Glacier Park during 1974 and 1975 respectively. In contrast, bighorn sheep herds in Glacier Park were sampled and found to have annual mean group sizes of 13.9 and 7.0 and aggregate indices of 22.7 and 15.1 during 1974 and 1975, respectively.
The precipitous nature of terrain favored by goats appears to set an upper limit on gregarious tendencies. That goats experience difficulty coordinating activities on steep cliff faces was confirmed by direct observation and by analysis of slope utilization, which showed that groups larger than 5 favored more moderate slopes than smaller groups. Large groups are also disadvantageous insofar as crowding under dangerously steep climbing conditions restricts maneuverability and forces all group members to share to some degree the genetic and experential limitations of the poorest mountaineers. Seton (1927) relates an incident in which a group of goats crowding onto a thin ledge and unable to turn back past one another eventually fell to their deaths.
Data from Brandborg (1955), Lentfer (1955), Kuck (1970), and Smith (1976) confirm small group size, consistently less than 5, throughout the year in this species. Group sizes were smallest in May as females separated from bands seeking isolation for the birth and early development of young. The largest groups were observed during summer. Increased group size at this time appeared most directly related to formation of nursery bands which increase defense capabilities and facilitate social interaction of neonates. Increasing tolerance of conspecifics by adult females was evident in nursery bands and contributed to their cohesion. Forage was abundant and readily accessible to all group members during summer months. Although large groups were also observed on winter range, it is significant that average group size did not substantially increase during winter despite confinement by deep snows of large numbers of goats to ranges of limited extent.
Group Composition
The tendency of subadults older than kids to continue following adult females can be measured by the very high percentages of yearlings and 2-year-olds in mixed groups (Table 15) in Glacier Park. The significance of leadership by the female adult class is emphasized by the lack of subadults in male groups. Groups containing only subadults were rare, never exceeding 3 percent of the population, even during May when parous adult females avoided other classes.
Table 15. Group association by class of mountain goats, Glacier National Park study area. Groups consisting solely of an adult female and kid were classified as solitary females.
| Class | Sample size |
Percent solitary |
Percent with subadults only |
Percent with males only |
Percent in mixed groups | |||||||||
| May | ||||||||||||||
| Yearling | 114 | 11.5 | 11.5 | - | 77.0 | |||||||||
| 2-yr-old female | 56 | 25.0 | 3.5 | 2.5 | 70.0 | |||||||||
| Adult female | 308 | 47.0 | - | - | 53.0 | |||||||||
| 2-yr-old male | 33 | 61.0 | - | 3.0 | 36.0 | |||||||||
| Adult male | 237 | 30.0 | 2.0 | 51.0 | 17.0 | |||||||||
| Average | 35.0 | 3.0 | 11.0 | 51.0 | ||||||||||
| June | ||||||||||||||
| Yearling | 43 | 7.0 | 5.0 | - | 88.0 | |||||||||
| 2-yr-old female | 19 | 10.5 | 5.0 | - | 84.5 | |||||||||
| Adult female | 199 | 34.0 | - | - | 66.0 | |||||||||
| 2-yr-old male | 18 | 22.0 | - | - | 78.0 | |||||||||
| Adult male | 137 | 55.0 | - | 27.0 | 18.0 | |||||||||
| Average | 26.0 | 2.0 | 5.0 | 67.0 | ||||||||||
| July | ||||||||||||||
| Yearling | 65 | 5.0 | 3.0 | - | 92.0 | |||||||||
| 2-yr-old female | 20 | 10.0 | 10.0 | - | 80.0 | |||||||||
| Adult female | 209 | 25.0 | - | - | 75.0 | |||||||||
| 2-yr-old male | 21 | 14.0 | - | 5.0 | 81.0 | |||||||||
| Adult male | 76 | 64.5 | - | 11.5 | 24.0 | |||||||||
| Average | 24.0 | 3.0 | 3.0 | 70.0 | ||||||||||
| August | ||||||||||||||
| Yearling | 52 | 4.0 | - | - | 96.0 | |||||||||
| 2-yr-old female | 37 | - | 3.0 | - | 97.0 | |||||||||
| Adult female | 290 | 20.0 | - | - | 80.0 | |||||||||
| 2-yr-old male | 21 | 28.0 | 5.0 | - | 67.0 | |||||||||
| Adult male | 125 | 28.0 | - | 43.0 | 29.0 | |||||||||
| Average | 16.0 | 2.0 | 8.0 | 74.0 | ||||||||||
| September | ||||||||||||||
| Yearling | 96 | 4.0 | - | - | 96.0 | |||||||||
| 2-yr-old female | 47 | 13.0 | 2.0 | - | 85.0 | |||||||||
| Adult female | 374 | 21.0 | - | - | 79.0 | |||||||||
| 2-yr-old male | 34 | 20.5 | 3.0 | 9.0 | 67.5 | |||||||||
| Adult male | 118 | 46.0 | - | 48.0 | 6.0 | |||||||||
| Average | 21.0 | 1.0 | 11.0 | 67.0 | ||||||||||
Two-year-old males were more often observed alone than 2-year-old females, and they were slightly better represented in predominantly male groups than were 2-year-old females. By 3 years of age, males assumed patterns of association which differed considerably from those of females and subadults. Adult males were more solitary than any other class. The majority of adult male social contacts occurred in small bachelor bands. The presence of adult males in mixed groups was occasional, and of relatively short duration in most instances. Outside the rut, adult males were most common in mixed groups during midsummer (Table 15).
Adult females were less gregarious than subadults. In Glacier Park, 47 percent occurred alone during May, and an average of 25 percent occurred alone from June through September. (Percentages of solitary females include those accompanied solely by their young.) Adult females were not observed in predominantly male groups.
The most common grouping in mountain goat herds was of an adult female with her young of the year followed by 1 or more subadults. Such typical group structures resemble "family" bands. However, evidence from marked animals suggests that while subadults and females maintain strong home range traditions, individual associations are highly transitory apart from the 10-11-month maternal-infant bond. Recognizable subadults and young adult females were seen to follow various older animals, often switching among several groups and leaders during the course of a day. Singer (1975) recorded marked individuals in the same group type only 21 percent of the time at a salt lick. I found marked and otherwise recognizable individuals to exhibit no consistent associations which might be interpreted as family bonds, although Chadwick (1974) and Smith (1976) have reported isolated instances of long-term female-subadult relationships. In general, then, herds appear to consist of loosely associated, interchanging bands. As might be expected, small, isolated wintering bands often possessed a relatively stable composition due to limited mobility and potential for exchange of members.
Distribution Within Habitats
High counts for each age and sex class were selected from repeated monthly surveys in 1974 and 1975 to obtain total counts of 314 and 300 goats, respectively, for the Glacier Park study area. Repeated surveys were assumed capable of locating about 80 percent of the existing population. Thus the actual number of goats was probably close to 360, establishing a density of 1.2 goats/km2 throughout the study area. Applying these population figures just to those habitats within the study area known to be utilized by goats, as defined in Fig. 4, densities of 2.8 goats/km2 on summer range and 5-10 goats/km2 on various winter ranges were derived.
On the 883-km2 portion of his study area situated within the southern part of Glacier Park, Singer (1975) estimated a population of 405 goats, yielding 0.46 goats/km2 (Fig. 1). Singer's study area contained a higher proportion of subalpine landforms and less extensive rock outcroppings than did mine, and apparently represented less optimal range situations. Sample surveys in inhabited goat range elsewhere in the park indicated that extrapolation of densities equivalent to those obtained by Singer and by myself was appropriate for similar adequate to optimal habitats, respectively. This resulted in a minimum parkwide population estimate of 1,500 mountain goats, nearly double traditional estimates exemplified by Cahalane's (1948) figure of 870. Park populations give every indication of being stable at present, and no historical evidence of major fluctuations was discovered.
Densities in the Swan Mountains were low: 0.15 goats/km2 throughout the 500-km2 study area. Due to the coincidence of summer and winter ranges, densities in summering habitats were only slightly lower than for winter ranges. Wintering densities varied from 0.13 to 1.5 goats/km2 for west-slope herds. The larger east-slope Bunker-Little Creek herd was confined to 2 discrete cliff areas where winter densities of 6.1-8.0 goats/km2 were recorded from 1971 through 1973 (Fig. 4).
Seasonal densities reflect both amount of available preferred habitat and degree of mobility permitted by existing snow conditions. Differences in monthly average intergroup distances in Glacier Park more than doubled from winter to summer conditions (Table 16). Table 17 presents monthly rates and extent of movements of goats in the Swan Mountains. Here, average distance traveled per feeding period increased five-fold from winter lows to summer, while average distance traveled per day increased more than twenty-fold.
Table 16. Average intergroup distance of mountain goat groups, Glacier Park study area, and monthly rate and extent of movements in the Swan Mountains study area. (From Chadwick 1974)
| N | Distance (m) | ||||||||||||||
| Jan | Feb | Mar | Apr | May | Jun | Jul | Aug | Sep | Oct | Nov | Dec | ||||
| Glacier Park | |||||||||||||||
| Average intergroup distance |
1974 | 626 | — | — | — | — | 221 | 421 | 577 | 425 | 470 | — | — | — | |
| 1975 | 492 | — | — | — | — | 288 | 462 | 484 | 571 | 405 | — | — | — | ||
| Swan Mountains | |||||||||||||||
| Distance traveled/ minute feeding |
4,570 | 1.5 | 1.1 | 0.6 | 0.6 | 1.5 | 3.5 | 3.5 | 3.1 | 2.5 | 1.2 | 1.1 | 0.5 | ||
| Average distanc traveled/feeding period |
4,570 | 33 | 69 | 141 | 114 | 117 | 163 | 144 | 124 | 161 | 138 | 59 | 30 | ||
| Average distance traveled/24 hours |
4,570 | 30 | 109 | 195 | 264 | 301 | 378 | 477 | 618 | 636 | 847 | 345 | 123 | ||
Table 17. Mountain goat social relationships outside the rutting season as defined by frequency of agonistic interaction and relative expression of dominant behavior between classes. Data are presented as number of interactions / percent behavior patterns expressing dominance. Percentages of dominant patterns between any 2 classes are not strictly complementary and usually exceed 100 percent since both participants often exhibited agonistic patterns while only one eventually exhibited submission during encounters.
| Recipient class |
Kid | Yearling | Two-year-old |
Adult | ||
| Male | Female | Male | Female | |||
| Kid | — | 65/100 | 35/100 | 31/100 | 1/100 | 104/99 |
| Yearling | 61/28 | 135/— | 70/93 | 105/89 | 0 | 164/100 |
| 2-yr-old male | 33/22 | 73/44 | 12/— | 41/41 | 9/80 | 121/70 |
| 2-yr-old female | 32/22 | 94/29 | 43/95 | 27/— | 8/38 | 129/100 |
| Adult male | 0 | 0 | 10/50 | 45/— | 70/48 | |
| Adult female | 88/4 | 179/10 | 139/49 | 119/11 | 69/— | 616/— |
SOCIAL ORGANIZATION
Leadership
Leadership is a subtle yet pervasive aspect of mountain goat society. Individual leaders generally determined type, tempo, and orientation of daily group activities. Leaders also directed movements between ranges and to salt licks. Behavior of leaders was the subject of considerable interest by group members while that of less important individuals, subadults in particular, was generally ignored. Gilbert (1974) found that older fallow deer (Dama dama) does wait longer in assessing situations, act with more deliberate and decisive movements, and are less reliant on the behavior of companions than other classes. A similar situation was found in the mountain goat. Studies of the amount of time expended by goats of different age and sex in surveying surroundings in an alert posture show a significant and regular increase in individual alertness with age and suggest some of the individual and group survival advantages of leadership by older animals, especially by adult females (Chadwick 1977).
Leadership was often associated with dominance, but the correlation was imperfect and difficult to distinguish from the influence of age, experience, and residual maternal-young patterns. Stewart and Scott (1948) reported a lack of correlation between leadership and dominance in the domestic goat (Capra hircus). Darling (1969:68-69) observed that leaders of hind groups in the red deer (Cervus elaphus) were generally older females but that "a female which ceases to be a regular breeder soon ceases to be a leader." Female mountain goats with young elicited stronger following responses than any other category and were followed even by those adult females to whom they were socially subordinate in agonistic encounters. Singer (1977) obtained similar results from goats using the Walton Lick in Glacier Park. Subadult animals displayed a reluctance to initiate movements on their own when in groups with adult females. In mixed groups, adult males generally followed adult females but exhibited marked independence of action in comparison with other classes. Young males followed older males in bachelor groups.
Lent (1974) stated that a major function of maternal behavior is the facilitation of learning processes in the infant by providing optimum levels of stimulation and a relatively stable social environment. Mountain goat kids were singularly inattentive to potential danger and dependent upon maternal direction, following within 20 m of their mother for nearly an entire year. Like maternal behavior, leadership relationships might be said to facilitate transmission of learned information concerned primarily with safety and efficient utilization of perennial home ranges to subadults.
Agonistic and Sexual Relationships
Mountain goat social interactions took place within a dominance hierarchy described by Geist (1964) and deBock (1970). As in most hierarchical societies, older and stronger individuals generally enjoyed higher ranks than younger, smaller animals. Aggressive adult females successfully dominated all other classes, while 2-year-old males, 2-year-old females, yearlings, and kids were increasingly subordinate (Table 17). The prolonged and highly developed following tendencies of kids seemed to be continually reinforced, since the further a kid strayed from its mother, the more it experienced displacement by dominant animals.
Relationships between males and females were complex and constituted an important departure from the linear age-size range structure described above. Aggressive behavior toward older animals was rarely exhibited by kids or yearlings except in the context of obvious play. Where sex identification was possible, those exceptions which did occur could nearly always be attributed to young males. Immature males tended to be slightly larger, more exploratory, and more aggressive than females of the same age. Males matured over the course of their second year and increasingly dominated adult females larger than themselves (Table 17). Two-year-old females, in contrast, remained almost entirely subordinate to adult females. Males first participating in the rut at 2.5 years of age directed mating behavior toward females of all ages though they were most successful at courting and dominating smaller, younger females (Chadwick 1974). After 2.5 years of age, mature males interacted with other classes almost exclusively in terms of sexual behavior during and outside the rut. As noted by Geist (1964), male sexual postures emphasize submissive elements and minimize threatening elements. In fact, males appeared to be inhibited from striking other classes while behaving in a sexual context and consequently began to "lose" contests with them in the sense that they would ultimately withdraw first. Opposing sexual and agonistic drives were expressed in unique male "conflict" postures and appeared to generate considerable stress in the male during social interactions.
Adult males observed in female-subadult groups were mainly young, 3-4 years of age. These males, having undergone a transition from agonistic to sexual modes of interaction with maturity, appeared to exert a destabilizing influence on mixed groups which led to elevated rates of aggression within the groups and frequently stressful conditions for the males themselves. Mature males thus became less able to interact successfully in female-subadult groups and eventually developed independent activity and range use patterns. Analysis of behavior (Chadwick 1977) revealed that while adult males were effectively subordinate to other classes at times, they did not exhibit typical submissive postures indicative of fear and/or flight. Adult males remained capable of dominating other classes when sufficiently motivated. This approach resolves some of the difficulties in interpreting male social relationships and explains why males are clearly dominant during the rut and in the presence of salt, as has been observed by Chadwick (1974), Rideout (1974), Singer (1975) and Moorhead (personal communication). The shift from agonistic to subordinate sexual behavior in maturing males did appear to neutralize potential for injury to other classes by the large, powerful billies. Of 3,916 extra-rut social interactions analyzed, only 199 involved actual horn weapon contact to one or both contestants, and none of these was initiated by an adult male.
Dominant animals were observed to preempt limited resources (pawed feeding craters, sheltered bedsites, and other areas favorable to energy conservation, salt, prospective mates, etc.) and control to various degrees the activities of lower-ranking individuals. Rank contests were seen to disrupt large groups on many occasions. In group situations, agonistic encounters excited onlooking goats, predisposing them to additional contests of their own. Redirected aggression by combatants also frequently involved adjacent group members. High-intensity battles were most common between individuals of similar rank (Chadwick 1977). Large groups were necessarily more likely to include individuals of similar status than small groups. The consequence of these different factors was that total number of agonistic encounters per group rose dramatically with increasing group size as did the number of agonistic encounters per individual in Glacier Park (Table 18) and the Swan Mountains (Table 19). Data from the two study areas are not quantitatively comparable due to different sampling techniques, but they reflect a similar pattern of increase. It became evident that high levels of aggression destabilized large groups, eventually causing members to separate into smaller units.
Table 18. Aggressive rates of mixed groups and male groups of different sizes during foraging, Glacier National Park study area.
| Group size |
Mixed groups |
Male groups | ||||
| Sample size |
Number of encounters/ group/hour |
Number of encounters/ individual/hour |
Sample size |
Number of encounters/ group/hour |
Number of encounters/ individual/hour | |
| 2 | 11 | 2.16 | 1.08 | 3 | 0 | 0 |
| 3 | 17 | 2.84 | 0.94 | 3 | 1.33 | 0.44 |
| 4 | 8 | 11.00 | 2.75 | 3 | 2.66 | 0.66 |
| 5 | 14 | 9.16 | 1.83 | 2 | 4.00 | 0.80 |
| 6 | 10 | 14.80 | 2.47 | 1 | 0 | 0 |
| 7-10 | 20 | 25.00 | 2.84 | 0 | — | — |
| >10 | 13 | 46.16 | 3.04 | 0 | — | — |
| Total | 93 | 12 | ||||
Table 19. Relationship of mountain goat group size to frequency of agonistic behavior while foraging, bedded, and licking salt, Swan Mountains study area.
| Group size |
Number of agonistic encounters/goat/hour | ||
| Foraging (N=1,055) | Bedded (N=549) |
Licking salt (N=43) | |
| 2 | 0.26 | 0.06 | 1.47 |
| 3 | 0.63 | 0.06 | 3.04 |
| 4 | 0.83 | 0.39 | 4.17 |
| 5 | 0.82 | 0.22 | 3.69 |
| 6 | 0.89 | 0.52 | — |
| 7 | 0.65 | 0.47 | — |
| 8 | 1.30 | 0.80 | 6.06 |
| 9 | 1.50 | 1.25 | — |
| 10 | 1.82 | 0.44 | — |
| >10 | 1.87 | 0.54 | — |
An increasing frequency and intensity of aggression acted to limit group size, agonistic relationships between classes placed similar constraints upon group composition. The result was distribution of herds within ranges as small, loosely associated bands of females and subadults and, separately, males. The most stable female-subadult bands were clearly defined linear hierarchies. Adult male associations appeared to be less aggressive than female-subadult groups outside the rut (Table 19). This helps explain the cohesiveness of certain bachelor bands observed in Glacier Park.
DISCUSSION
Three major points proceed from evidence demonstrating occupation of a highly specialized topographic niche by mountain goats. First, because of terrain requirements, habitats are selected from within a narrow range of stable periglacial alpine and subalpine plant communities. Secondly, in a topographic rather than vegetational context, mountain goats occupy habitat with dense cover qualities which provide a high degree of seclusion and escape potential. In northern biomes, the impact of predation on artiodactyls is most significant during winter. However, use of precipitous rock outcroppings by goats increased under winter conditions, mitigating predation as a cause of mortality. Third, mountain goat habitat selection appears to minimize competition from sympatric artiodactyls.
These points argue that the carrying capacity of mountain goat range is fundamentally determined by extrinsic abiotic factors, mainly climate and primary (geomorphic) succession, while biotic factors of secondary (plant) succession, predation, and competition seem not to be limiting. Mountain goat populations appear to occupy the extreme upper-elevational limits of faunal distribution over most of their continental range. On an environmental gradient from complete biological accommodation to complete control by physical phenomena, mountain goats must be placed well toward the physically controlled end. Whereas biotic pressures from competitors and predators may have originally driven rupicaprid ancestors of Oreamnos to exploit high, precipitous terrain, we would expect much of their evolution since that time to have occurred in response to the physical demands of that niche.
In addition to innate climbing abilities and obviously adaptive morphology, including specialized pelage, flexible traction-pad hooves, short cannon bones, extreme development of anterior musculature, bilateral flattening of body, and skeletal configuration placing front and rear hooves in close proximity, behavioral adaptations have also evolved in response to physical environmental demands. Plasticity of diet (Geist 1962) coupled with flexible foraging style and transmission of learned information regarding resources permit the mountain goat to achieve high densities in its niche, as do size, structure, and distribution of social units.
Ledge habitats, for which goats expressed strong preference in all months and utilized almost exclusively two-thirds of the year under winter conditions, support a discontinuous food supply. This food supply is typically distributed as an irregular series of thin strips separated by rock and talus. Jarman (1973) correlated small group size with patchy food resources in a comparison of African bovids. Houston (1974) related the semi-solitary habits of moose to a highly dispersed winter food supply. Schaller (1973) found that Himalayan tahr (Hemitragus jemlahicus) had an average group size of 6.5 compared to 23.0 in Nilgiri tahr (H. hylocrius), and pointed out that the Himalayan species utilizes narrow cliff ledges while the Nilgiri species is found on rolling grasslands. Average group size in the mountain goat populations studied was small, with a high proportion of the populations occurring alone or in pairs. Annual mean group sizes and aggregate indices for mountain goats in Glacier Park were considerably smaller than for bighorn sheep, which utilized more continuous grassland habitats just below goat ranges. It was also demonstrated that goats utilizing moderate slopes, such as those on which sheep might be observed, occurred in larger groups than those on precipitous terrain. It therefore appears that the semi-gregarious nature of mountain goat society is primarily an adaptation to the nature of the food supply within their topographic niche.
Given the dispersed and highly localized nature of critical winter forage and confinement to microniche food sources for long periods during storms, individuals and small groups might obtain adequate nutrition and shelter where a larger group would quickly exhaust available food and force many group members to use poor-quality shelter. Small group size also facilitates coordination of travel and other band activities in precipitous terrain, disperses total zootic impact on fragile plant communities, and confers some immunity from avalanches. Avalanches were shown to be a major agent of mortality which might have in itself provided selective pressure toward less gregarious social tendencies. Holroyd (1967) stated, "The fact that they [mountain goats) are seldom closely bunched would probably result in only one or two being taken by a single slide rather than large numbers."
Size and structure of mountain goat groups were found to be mediated by agonistic behavior. In the absence of significant interspecific competition, predation, or successional vegetation influences, we would expect development of complex social mechanisms to structure intraspecific competition and maintain total population size within the carrying capacity. It was shown that both summer and winter food habits incorporated frequent and often extensive vertical and lateral movements. The discontinuous and unpredictable nature of the food supply thus favored development of a mobile system of dominance, or hierarchy, rather than a fixed territorial system. The frequency and intensity of agonistic behavior observed in mountain goats, especially high among females and subadults, probably reflects the species' extreme location on the biologically accommodated, physically controlled gradient.
Competition between adult males and the female-young population component was minimized as mature males sought peripheral or separate ranges to avoid social stress. Adult males generally yielded personal space to females and even to subadults when in contact with them. Foss (1962) received the impression that adult males in the Crazy Mountains, Montana, occupied "rougher" terrain than other classes. DeBock (1970), Chadwick (1974), Rideout (1974), Smith (1976), and Kuck (personal communication) observed that many adult males occupy habitats judged to be marginal in comparison with those of females and subadults. Use of suboptimal range may partially explain why adult male mountain goats are usually less numerous in most populations than adult females, as summarized by Hibbs (1966) and Vaughan (1975).
Competition between adult females and juveniles was structured to the consistent advantage of the productive female class. Under conditions of scarcity, dominant animals in stable groups enjoyed relatively uncontested access to resources. This had the overall effect of standardizing the resource base of dominant animals while magnifying resource shortages to subordinates. Consequently, adult survival appeared constant, particularly in the generative female class, while juvenile mortality varied considerably under different winter regimes. Natality, which also reflects the resource base available to adult females, is expected to vary substantially in optimal range situations only when winter conditions are so extreme as to significantly alter extent of available range. Moderate fluctuations in the winter regime between years should not greatly alter natality in optimum ranges but should have a more measurable impact on natality in marginal ranges. Production of young by 3-year-old females is expected to be lower and more responsive to winter conditions than that of older females. Disparities between 3-year-old female natality and older adult female natality should be most apparent on marginal ranges.
Natality was quite constant in the Glacier Park study area, while limited data indicate that natality indeed varied with winter conditions in the Swan Mountains. Hjeljord (1973) observed unusually low production of young following a particularly severe winter, as did Brandborg (1955). Rideout (1974) found that natality varied with winter conditions in the marginal Sapphire Mountains, Montana, range as did Smith (1976) in the Bitterroot Mountains, Montana.
Loss of over 50 percent of the kid cohort during their first winter has been reported in other native goat populations (Anderson 1940, Cowan 1944, Brandborg 1955). By distinguishing 2-year-old classes, it was found that similarly high losses may also occur in the year ling class. Over the winter of 1974-75, percent yearling losses exceeded those of the kid class in Glacier Park.
Typical winter weights of kids and yearlings are approximately 18 and 27 kg, respectively (Brandborg 1955). Juvenile animals with high metabolic requirements, low fat reserves, and high surface-to-volume ratios suffer thermal disadvantages which in themselves would lead to much higher mortality rates than in adults under winter stress, particularly in the kid class. Gains in weight and experience from kid to yearling class are partially offset by loss of maternal protection and consequent competitive disadvantages within group hierarchies.
Both Geist (1966) and Klein (1968) noted a relationship between the self-regulating ability of species and stability of vegetation communities in which they evolved. Although fire may play a larger role in the ecology of coastal mountain goat populations where many winter ranges are within the forest zone, stable ledge habitats on rock outcroppings remain of prime importance. Given the mountain goat's use of traditional home range in these spatially limited, topographically defined habitats; poorly developed colonizing abilities; and the fragile, slow-growing characteristics of high-elevation plant communities, it would be particularly disadvantageous for mountain goats to exceed and thereby reduce the carrying capacity of their range. It therefore seems likely that as increasing niche specialization led to reduction of biotic regulating influences, social mechanisms evolved which distribute range use, structure intraspecific competition, and cause density-influenced mortality consistent with physical environmental stresses and a recurrently scarce food supply.
LITERATURE CITED
Anderson, N. A. 1940. Mountain goat study. Washington Dept. of Game Biol. Bull. 2. 21pp.
Bansner, U. 1974. Mountain goat-human interactions in the Sperry-Gunsight Pass area of Glacier National Park. Prog. Rep. Univ. of Montana, Missoula. 38pp. (Mimeogr.)
________. 1976. Mountain goat-human interactions in the Sperry-Gunsight Pass area of Glacier National Park. Univ. of Montana, Missoula. 46pp. (Mimeogr.)
Brandborg, S. M. 1955. Life history and ecology of the mountain goat in Idaho. Idaho Dept. of Fish Game Wildl. Bull. 2. 142pp.
Cahalane, V. J. 1948. The status of mammals in the U.S. National Park system. J. Mammal. 29:247-259.
Casebeer, R. L. 1948. A study of the food habits of the mountain goat (Oreamnos americanus missoula) in western Montana. M.S. Thesis. Montana State Univ., Missoula. 84pp.
_________, M. J. Rongrud, and S. Brandborg. 1950. The Rocky Mountain goat in Montana. Montana Fish Game Commission Bull. 5. 107pp.
Chadwick, D. H. 1974. Mountain goat ecology-logging relationships in the Bunker Creek drainage of western Montana. M.S. Thesis. Univ. of Montana, Missoula. 262pp.
_________. 1977. The influence of mountain goat social relationships on population size and distribution. Proc. Int. Mountain Goat Symp. 1. (In press)
Cowan, I. McT. 1944. Report of wildlife studies in Jasper, Banff, and Yoho National Parks in 1944 and parasites, diseases, and injuries of game animals in the Rocky Mountain national parks, 1942-1944. Canadian Wildlife Service, Ottawa. 83pp. (Mimeogr.)
Craighead, J. J., M. G. Hornocker, M. W. Shoesmith, and R. I. Ellis. 1969. A marking technique for elk. J. Wildl. Manage. 33(4):906-909.
Darling, F. F. 1969. A herd of red deer. Oxford University Press, London. 215pp.
DeBock, E. A. 1970. Behavior of the mountain goat. M.S. Thesis. Univ. of Alberta, Edmonton. 173pp.
Dyson, J. L. 1949. Glaciers and glaciation in Glacier National Park. Glacier Nat. Hist. Assoc. Spec. Bull. 3. 24pp.
Foss, A. J. 1962. A study of the Rocky Mountain goat in Montana. M.S. Thesis. Montana State Univ., Bozeman. 26pp.
Geist, V. 1962. Observations on the habitat-directed behavior of Stone's sheep (Ovis dalli stonei) and the mountain goat (Oreamnos americanus). Proc. Alaskan Sci. Conf. 13:29-30.
_________ 1964. On the rutting behavior of the mountain goat. J. Mammal. 45(4):551-568.
_________. 1966. On the behaviour and evolution of American mountain sheep. Ph.D. Thesis. Univ. of British Columbia, Vancouver. 251pp.
_________. 1967. On fighting injuries and dermal shields of mountain goats. J. Wildl. Manage. 31(1):192-194.
_________. 1971. Mountain sheep: a study in behavior and evolution. University of Chicago Press, Chicago. 383pp.
Gilbert, B. K. 1974. The influence of foster rearing on adult social behavior in fallow deer (Dama dama). IUCN Publ. New Ser. 24:247-273.
Habeck, J. R. 1970. The vegetation of Glacier National Park, Montana. Univ. of Montana Spec. Rep. 132pp.
Hanson, W. O. 1950. The mountain goat in South Dakota. Ph.D. Thesis. Univ. of Michigan, Ann Arbor. 92pp.
Hibbs, L. D. 1966. A literature review on mountain goat ecology. Colorado Dept. Game, Fish, Parks and Colorado Coop. Wildl. Res. Unit Spec. Rep. No. 8. 23pp.
_________. 1967. Food habits of the mountain goat in Colorado. J. Mammal. 48(2):242-248.
Hjeljord, O. 1971. Feeding ecology and habitat preference of the mountain goat in Alaska. M.S. Thesis. Univ. of Alaska, Fairbanks. 126pp.
_________. 1973. Mountain goat forage and habitat preference in Alaska. J. Wildl. Manage. 37(3):353-362.
Hoefs, M. E. G. 1974. Food selection by Dall's sheep Ovis dalli dalli. IUCN Publ. New Ser. 24:759-786.
Holroyd, J. C. 1967. Observations of Rocky Mountain goats on Mt. Wardle, Kootenay National Park, B.C. Can. Field-Nat. 81:2-22.
Houston, D. B. 1974. Aspects of the social organization of moose. IUCN Publ. New Ser. 24:690-696.
Jarman, P. J. 1973. The social organization of antelope in relation to their ecology. Behaviour 46:215-271.
Johnston, A., L. M. Bezeau, and S. Smoliak. 1968. Chemical composition and in vitro digestibility of alpine tundra plants. J. Wildl. Manage. 32(4):773-777.
Karaer, G. J. 1961. Reindeer fodder resources. In P. S. Zigunov, ed. Reindeer husbandry. Chapter 4. English translation. U.S. Dept. of Commerce Clearinghouse for Federal Scientific and Technical Information, Springfield, Va.
Kessell, S. R. 1976. A gradient vegetation, resource, and fire management model for Glacier National Park, Montana. Ph.D. Thesis. Cornell Univ., Ithaca, N.Y. 578pp.
Klein, D. R. 1968. The introduction, increase, and crash of reindeer on St. Matthew Island. J. Wildl. Manage. 32(4):350-367.
_________. 1970. Food selection by North American deer and their response to over-utilization of preferred plant species. In A. Watson, ed. Animal populations in relation to their food resources. Blackwell Scientific Publications, Oxford.
Kuck, L. 1970. Rocky Mountain goat ecology. Idaho Fish Game Dept. Proj. Compl. Rep., Proj. W-144-R-1. 37pp.
Lent, P. C. 1974. Mother-infant relationships in ungulates. IUCN Publ. New Ser. 24:14-55.
Lentfer, J. W. 1955. A two-year study of the Rocky Mountain goat in the Crazy Mountains, Montana. J. Wildl. Manage. 19(4):417-429.
Matthes, F. E. Glaciers. Pages 190-215 in Hydrology: physics of the earth. McGraw-Hill Book Co., New York.
Oelberg, K. 1956. Factors affecting the nutritive value of range forage. J. Range Manage. 9:220-225.
Peck, S. V. 1972. The ecology of the Rocky Mountain goat in the Spanish Peaks area of southwestern Montana. M.S. Thesis. Montana State Univ., Bozeman. 54pp.
Petrides, G. A. 1948. Mountain goat age ratios in Montana. J. Mammal. 29(2):185.
Ream, R., R. R. Beall, and L. Marcum. 1971. Sapphire Range elk ecology study: elk, logging and people. Univ. of Montana School of Forestry Annu. Rep. 28pp.
Rideout, C. B. 1974. A radiotelemetry study of the ecology and behavior of the mountain goat in western Montana. Ph.D. Thesis. Univ. of Kansas, Lawrence. 146pp.
Ross, C. B. 1959. Geology of Glacier National Park and the Flathead region of northwestern Montana. U.S. Geol. Surv. Pap. No. 296.
Saunders, J. K., Jr. 1955. Food habits and range use of the Rocky Mountain goat in the Crazy Mountains, Montana. J. Wildl. Manage. 19(4):429-437.
Schaller, G. B. 1973. Observations on Himalayan tahr. J. Bombay Nat. Hist. Soc. 70(1):1-24.
Seton, E. T. 1927. Lives of game animals. Vol. 3. Doubleday Publ. Co., New York. 780pp.
Singer, F. J. 1975. Behavior of mountain goats, elk, and other wildlife in relation to U.S. Highway 2, Glacier National Park. Federal Highway Administration, Denver. 96pp.
_________. 1977. Dominance, leadership and group cohesion of mountain goats at a natural lick, Glacier National Park, Montana. Proc. Int. Mountain Goat Symp. 1. (In press)
Smith, B. L. 1976. Ecology of Rocky Mountain goats in the Bitterroot Mountains, Montana. M.S. Thesis. Univ. of Montana, Missoula. 203pp.
Stewart, J. C., and J. P. Scott. 1948. Lack of correlation between leadership and dominance relationships in a herd of goats. J. Compar. Physiol. Psychol. 40:255-264.
Vaughan, M. R. 1975. Aspects of mountain goat ecology, Wallowa Mountains, Oregon. M.S. Thesis. Oregon State Univ., Corvallis. 113pp.
chadwick/index.htm
Last Updated: 8-Apr-2013