|
NATIONAL PARK SERVICE
Research in the Parks NPS Symposium Series No. 1 |

|
Ecology of the Saguaro: I. The Role of Freezing Weather in a Warm-Desert Plant Population
WARREN F. STEENBERGH and CHARLES H. LOWE National Park Service and University of Arizona, Tucson
"The line which marks the extreme southern limit of frost is the most important climatic boundary in restricting the northward extension of perennial tropical species, and it is the line along which the influence of winter cold is the simplest in its operation."—Forrest Shreve, The Influence of Low Temperatures on the Distribution of the Giant Cactus, 1911:136.
INTRODUCTION
The saguaro giant cactus (Cereus giganteus Engelm., Carnegiea gigantea [Engelm.] Britt. and Rose) is the northernmost species in a large group of tropical columnar cacti. Herein lies its principal problem at Saguaro National Monument, a problem shared with many tropical and subtropical plant species in a locality subjected to yearly subfreezing weather (Fig. 1).

|
| Fig. 1. Winter at Saguaro National Monument, Tucson, Arizona. National Park Service photo by George Olin, 17 November 1958. |
As a major plant dominant in a subtropical desert, the saguaro is a species with the majority of its geographic distribution to the south in Mexico, where it occurs throughout the mainland Sonoran Desert into the subtropical deciduous forest of southern Sonora (Fig. 2). Accordingly, its distribution occurs both north and south of the "freezing line" in central Sonora. At Tucson, the population occurs in the northeastern sector of the species distribution. At Saguaro National Monument (east), it occurs at the edge of the desert for, contrary to popular belief, Tucson does not lie in the heart of the Sonoran Desert. As we will develop throughout this paper, the saguaro population at Saguaro National Monument is periodically subjected to catastrophic selection during the winter, with widespread freeze-kill throughout the population. This has become fully evident through our recent studies. Heretofore, however, and for many years, the widespread mortality of saguaros has been attributed to other causes in spite of the early observations of workers at Tucson who pointed directly to the problem (Shreve 1911; Thornber 1911, 1916; Turnage and Hinckley 1938; and others).
|
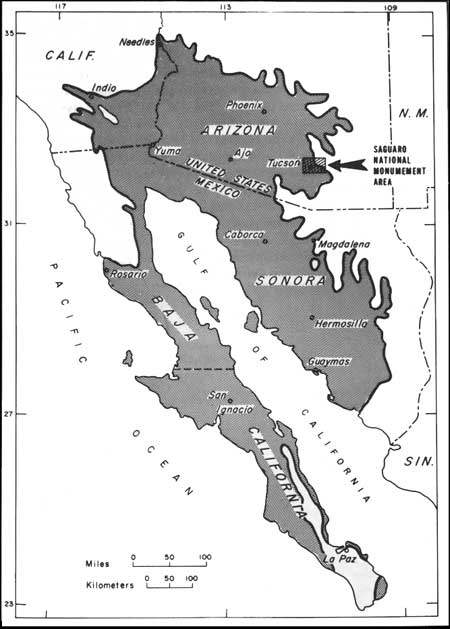
| Fig. 2. The Sonoran Desert showing the Saguaro National Monument area at Tucson, Arizona (after Shreve 1951). The eastern portion (Rincon Mountain Section) of the Monument lies at the northeastern boundary of the Sonoran Desert. The western portion (Tucson Mountain Section) is located within, but close to the edge of, that boundary. |
What is happening and has been happening to the saguaro at Saguaro National Monument is really no mystery and is certainly not a secret, for density-independent climatic regulators are common knowledge to all ecologists. In discussing the natural regulation of populations in general, Harper (1967) could have been speaking precisely of the monument saguaro situation: "In environments in which there is a recurrence of natural hazards, populations may spend most of their time recovering from the hazards . . . . The size of such populations may frequently be a function of the magnitude of the last catastrophe and the time available for recovery." The observations and experiments reported here confirm Harper's conclusion and apply to the saguaro population at Saguaro National Monument.
Recent reports (since 1962) on saguaro ecology include evaluation on climatic influences and historical factors (Niering et al. 1963; Hastings and Turner 1965), and mortality and survival of young saguaros (Turner et al. 1966; Steenbergh and Lowe 1969; Turner et al. 1969). Seedling respiration physiology is reported by Despain et al. (1970), germination responses by McDonough (1964), and cristation by Snyder and Weber (1966). Steelink et al. (1967) and Steelink et al. (1968) have reported on saguaro tissue chemistry, and distribution and taxonomic characteristics are included in publications by Earle (1963) and by Benson (1969).
This report on saguaro winter mortality and other investigations on the ecology of the saguaro cactus reported elsewhere (Lowe 1966; Soule and Lowe 1970; Steenbergh 1970, 1972; Steenbergh and Lowe 1969) are the result of a continuing National Park Service program of research designed to provide scientific information to facilitate the management and interpretation of Saguaro National Monument.
Field investigations were conducted in the two sections of the Saguaro National Monument near Tucson, Arizona (Fig. 2). The eastern portion (Rincon Mountain Section), situated 15 miles east of Tucson, lies along the northeastern boundary of the Sonoran Desert. The western portion (Tucson Mountain Section), located 15 miles west of Tucson, is within but close to the edge of that boundary. Elevations range from 823 m (2700 ft) to 2641 m (8666 ft) in the eastern section and from 616 m (2020 ft) to 1429 m (4687 ft) in the western section. New data reported here were obtained from December 1967 to December 1971 and include earlier observations by the authors and others on the saguaro population at Saguaro National Monument.
MATERIALS AND METHODS
Data reported here on survival and freeze-caused mortality in natural populations of saguaro cactus in both sections (east and west) of Saguaro National Monument were obtained from 3 January to 21 November 1971. Observations in 1971 were initiated concurrently with the onset of a period of subfreezing nocturnal temperatures from 3 to 10 January at Tucson Airport (U.S. Weather Bureau 1971).
Data on a sample population of 189 saguaros (height 0.09-13 m) was obtained from a 2-ha (100 x 200 m) permanent sample plot located at Saguaro Monument (west) flats (Fig. 13). All saguaros within the plot were numbered and provided with identification tags. Height of each plant, the number of arms, and the general condition of each individual were noted, together with the nature and extent of any visible freeze-caused damage. Stem-height measurements of all plants were made, using two specially designed height-guages for plants up to 6.8 m tall, and a Haga altimeter for larger plants. Heights of plants to 2 m were recorded to the nearest whole millimeter; 2-6.8 m plants, to the nearest centimeter; plants over 6.8 m, to the nearest decimeter (Table 12; Fig. 21). In addition, heights were measured, and observations on the condition of wild, previously located juvenile plants adjacent to that plot and at Saguaro Monument (east) flats (N=129) were made at irregular intervals to 21 November (Table 10, Fig. 19). Concurrent observations at Saguaro National Monument (east and west) on both wild plants and naturally growing survivors from seed-broadcasting and winter-mortality experiments of previous years were employed to obtain information concerning winter mortality of known-age (1st to 7th year) globose juvenile plants germinated 1964-70 (Tables 6, 7, 8, 9; Figs. 3, 4, 5, 15, 16, 17, 18).
As used in this paper, seedlings refer to plants less than 1 year old, juveniles are plants over 1 year old that have not reached flowering size (ca. less than 2 m height), and adults are flowering plants, generally over 2 m in height. Juveniles we further subdivide by age-related form into globose juvenile plants (heights to ca. 2.5 cm, age 1 to 6-7 years) with nippled (tuberculate) stems, and larger columnar juveniles plants with evidently fluted (ribbed) sterns (Figs 3, 4, 5, 6, 7).

|
| Fig. 3. Saguaro seedling, age 3 months, stem height approximately 2 mm. The pointer on the left is a common dressmaker's pin. The first year of life (first 12-14 months)—from germination to the beginning of the second summer's growth—is the critical period of saguaro establishment. Germinated 7 August 1971, photographed 4 October 1971 by Dana Slaymaker. |

|
| Fig. 4. Globose juvenile saguaro, age 13 months, stem height approximately 7 mm. At this age this saguaro has survived the hazards that kill nearly all of the seedlings that germinate each summer. Photographed 13 August 1970 by Harold T. Coss. |

|
| Fig. 5. Globose juvenile saguaro, age 26 months, height approximately 1 cm. Plants of this form (age 1 to ca. 7 years, heights to ca. 2.5 cm) are highly vulnerable to freeze-caused injury and death. Photographed 13 August 1971 by Harold T. Coss. |

|
| Fig. 6. Columnar juvenile saguaro, height 12.5 cm. Resistance of such plants to death from freezing increases with age-related changes in size, volume, and form. Photographed 19 January 1971. |

|
| Fig. 7. Young adult saguaro, height 2.1 m (7 ft) in first or second year of flower production. Saguaros with this form (approximately 1.0-3.5 m height; 3-11.5 ft) are relatively freeze resistant. Photograph 19 June 1965. Most saguaro deaths from catastrophic freezing occur in smaller juvenile and larger adult size classes of the population. |
Periodic observations 3 January to 2 August 1971 on seedlings surviving from 1968 seed-broadcasting experiments in natural microenvironments were made to obtain survivorship data on 30-month-old plants at Saguaro Monument (east) flats (Table 6; Fig. 15). Observations were made to 8 April 1971 for data on comparative survival in different habitats (Table 7; Figs. 13, 14, 16), and for shielded and unshielded microenvironments (Table 8; Fig. 17). Unshielded plots were covered with round (61 cm dia. X 30 cm ht.) 0.5-inch mesh (1.3 cm) hardware—cloth exclosures; shielded plots were covered with 16 mesh (0.16 cm) window-screen exclosures of identical size.
Continuing previously reported studies on critical factors in saguaro establishment and survival (Steenbergh and Lowe 1969), the experiment utilizing transplanted juvenile saguaros to obtain additional information on minimum temperatures and winter mortality of saguaros in differing types of habitats and microenvironments was initiated in December 1967. Saguaros used in the experiment were germinated during the summers of 1964 and 1965, and grown outdoors under partial shade in wooden flats containing native soil. Seedlings were provided with supplemental water during the first growing season, and received water only from natural rainfall thereafter.
On 12 and 15 December 1967, the height and diameter of each individual were measured with calipers, and four 42-month-old plants (class of 1964) and sixteen 30-month-old plants (class of 1965) were transplanted to each of nine study sites (N = 20 plants per site, Table 2). Height of 1964 plants (N = 36) was 20.14 mm (±0.38), with range 16-24 mm; diameter was 18.44 mm (±0.40). Height of 1965 plants (N = 144) 10.61 mm (±0.16), range 7-16 mm; diameter was 9.22 mm (±0.21), range 6-14 mm. In each plot, transplants were spaced approximately 10 cm apart in five rows of four plants each, lightly watered, and the plot was covered with a 0.5-inch mesh hardware-cloth cage (30 cm ht. X 61 cm dia.) to exclude mammals and birds. Taylor maximum-minimum thermometers were installed at each site on 20 December. Each thermometer, provided with a cylindrical condensation shield (6.5 X 16-cm tin can with ends removed), was placed horizontally adjacent to the cage with a sensing bulb approximately 2 cm above the soil surface. At approximately 1-week intervals beginning 3 January, the indicated minimum temperature and the condition of all saguaros at each site were recorded (Tables 2, 3, 4; Figs. 9, 10, 11, 12).
Thermometers at the Saguaro Monument (east) flats open (SEFO) and tree-crown canopied (SEFC) sites were destroyed by vandals after the 10 January observation. Mean and 21 February temperatures for these two sites (shown in Table 2; Figs. 9, 10) were estimated using the 3 and 10 January observations and from nearby thermometer readings.
During the winter of 1968-69, further data on winter mortality was obtained using outdoor, pot-grown, first-year seedlings at three of the same Saguaro Monument (east) habitats used the previous winter (rocky north-facing slopes, rocky south-facing slopes, and flats). Seedlings which germinated about 8 September 1968, in waxed card board tubs (6 X 9 cm) provided with bottom drainage and filled with native soil, were grown outdoors in partial shade. Seedlings were watered weekly until mid-November when they had reached approximately the same size as first-year wild seedlings (ca. 3 mm above-ground stem height, Fig. 3). Seedlings received water only from natural rainfall after 15 November. On 13 December a tub with rooted seedlings intact was "planted" in a shallow excavation at each experimental site in the natural manner, i.e., with seedlings and the soil surface within the tub all flush with the natural ground surface. Seedlings in each tub were counted (Table 1) and covered with a single layer of Armex Polyprophylene Shading Fabric (46% shade) to prevent possible sunscald injury. Each plot was then covered with a 30 X 61-cm, 0.5-inch mesh hardware-cloth cage and a Taylor self-registering thermometer installed as in the 1967-68 winter experiment. Observations on temperature and seedling condition were also carried out in the same manner as during the previous winter (Table 1; Fig. 8).
|
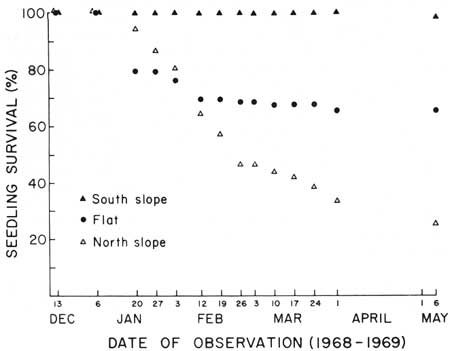
| Fig. 8. Differential winter survival of first-year saguaro seedlings in three types of habitat at Saguaro National Monument, east, 13 December 1968 to 6 May 1969. Symbols indicate percent of the original population surviving on the date of observation in flat (circles, N = 92), rocky south slope (solid triangles, N = 109), and rocky north slope (open triangles, N = 107) habitats. Data in Table 1. All seedling deaths occurred as a result of freezing. Progressive die-off of seedlings occurs as a delayed response to different degrees of simultaneously occurring critical injuries from freezing. |
Tucson temperature data at the University of Arizona Station graphed in Fig. 25 are from U.S. Weather Bureau Climatic Data Summaries for Tucson, Arizona (1894-1971). There and elsewhere in this paper, temperatures are reported as recorded in degrees Fahrenheit to simplify presentation of the data and facilitate comparisons here.
Winter measurements (December) referred to here for nocturnal radiative shielding by natural vegetation canopies and artificial covers were recorded with a Stoll-Hardy infrared radiometer. The saguaro supercooling limits reported were determined experimentally by continuous recording of the stem-core temperature during cooling, supercooling, and freezing. Tissue implant thermistor probes (calibrated, ±0.01°C) were imbedded in intact saguaros placed singly in pyrex respiration chambers and submerged in an ethylene-glycol water bath cooled to subfreezing temperatures. Additional samples were monitored with special fine bulb, rapid-adjusting thermometers in constant temperature cold-rooms held at subfreezing temperatures.
Age-height relationships, shown in Table 13 and Fig. 24, are based on saguaro growth curves for plants less than 1 m high from on-going investigations by the authors and studies reported by Shreve (1910) and Hastings and Alcorn (1961).
RESULTS AND DISCUSSION
Freezing weather was the primary cause of winter deaths in all populations of known-age seedlings and juvenile saguaros observed during this investigation. Experimental data are provided. Insects were the only other observed cause of winter mortality of young saguaros.
The 1968-69 Experiment
First-year saguaro seedling survival and associated minimum temperatures in rocky north slope, in rocky south slope, and in flat habitats are shown in Table 1 and Fig. 8. Differential survival associated with differences in the winter thermal environments of each of these three types of habitats is critical to seedling establishment, i.e., survival from germination to the beginning of the second year of life.
TABLE 1. Winter minimum temperatures and survival of first-year saguaro seedlings in three types of habitat at Saguaro National Monument (east). 13 December 1968 to 6 May 1969. Temperatures are the lowest minimum temperature (°F) recorded during the interval since the last observation. Data graphed in Fig. 8.
| Date (1968-69) |
Rocks — S. Slope |
Rocks — N. Slope |
Flats | ||||||
| Survivors | Survivors | Survivors | |||||||
| No. | Percent | Min. T | No. | Percent | Min. T | No. | Percent | Min. T | |
| 13 December | 109 | 100.0 | 107 | 100.0 | 92 | 100.0 | |||
| 31 December | 109 | 100.0 | 22 | 107 | 100.0 | 19 | 92 | 100.0 | 17 |
| 6 January | 109 | 100.0 | 30 | 107 | 100.0 | 26 | 92 | 100.0 | 27 |
| 20 January | 109 | 100.0 | 40 | 101 | 94.4 | 36 | 73 | 79.4 | 32 |
| 27 January | 109 | 100.0 | 40 | 93 | 86.9 | 38 | 73 | 79.4 | 38 |
| 30 January | 109 | 100.0 | 26 | 93 | 86.9 | 24 | 73 | 79.4 | 21 |
| 3 February | 109 | 100.0 | 27 | 86 | 80.4 | 24 | 70 | 76.1 | 22 |
| 12 February | 109 | 100.0 | 34 | 69 | 64.5 | 29 | 64 | 69.6 | 29 |
| 19 February | 109 | 100.0 | 36 | 61 | 57.0 | 35 | 64 | 69.6 | 31 |
| 26 February | 109 | 100.0 | 31 | 50 | 46.7 | 31 | 63 | 68.5 | 29 |
| 3 March | 109 | 100.0 | 32 | 50 | 46.7 | 31 | 63 | 68.5 | 29 |
| 10 March | 109 | 100.0 | 31 | 47 | 43.9 | 31 | 62 | 67.4 | 26 |
| 17 March | 109 | 100.0 | 28 | 45 | 42.1 | 28 | 62 | 67.4 | 26 |
| 24 March | 109 | 100.0 | 36 | 41 | 38.3 | 37 | 62 | 67.4 | 33 |
| 1 April | 109 | 100.0 | 37 | 36 | 33.6 | 36 | 60 | 65.2 | 33 |
| 6 May | 107 | 98.2 | 27 | 25.2 | 60 | 65.2 | |||
The observable ultimate death of young plants may be delayed for a period of weeks or months after the occurrence of critical subfreezing temperatures. The length of time from critical freeze-caused injury to observable death depends upon the severity of the original injury and the directly resulting desiccation associated with warming temperatures during late winter and early spring. The severity of freeze-caused injury may be increased by subsequent occurrences of subfreezing temperatures. In summary, during this period of observation (5 months), 37% of all saguaros in the sample died from freezing.
The 1967-68 Experiment
Winter minimum temperatures and associated survival and mortality of 30- and 42-month-old juvenile saguaros (N = 180) at Saguaro National Monument during the winter of 1967-68 are shown in Tables 2 and 3 and Figs. 9 and 10. Freeze-caused damage was first observed on 3 January 1968, following the occurrence of critically low temperatures between 20 December and 3 January. Deaths resulting from these injuries were first confirmed on 17 January and continued through 27 March when the last freeze-caused death was recorded.
|
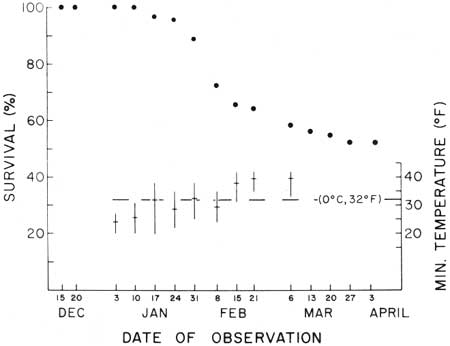
| Fig. 9. Winter survival summary for known-age (30 and 42 months) juvenile saguaros (N = 180) at nine sites, Saguaro National Monument, 15 December 1967 to 3 April 1968, and associated ground-level minimum temperatures from 20 December to 6 March. Circles indicate percent of plants living on date of observation. Temperatures shown are the mean (cross-bar) and range (vertical bar) of the extreme minimum temperatures recorded at individual sites during the period between each observation. Temperatures are reported for all sites (N = 9) to 10 January and seven sites thereafter (no subsequent record available thereafter for two sites at Saguaro Monument, east, flats). Data in Tables 2 and 3. The ultimate collapse and observable death of young plants critically damaged by subfreezing January temperatures continued through February and March. |
|
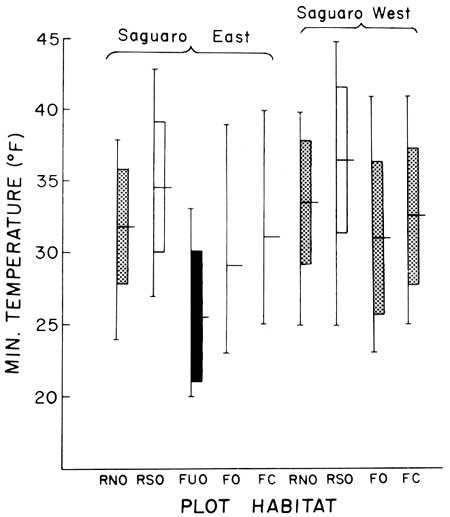
| Fig. 10. Minimum temperatures (°F) at nine experimental sites, Saguaro National Monument, 20 December 1967 to 6 March 1968. Range, mean, and 95% confidence interval for the ground-level minimum temperatures at each site (N = 9 observations, see Table 2). Minimum temperatures at both rocky south-facing slope sites (white bar) are significantly different (P < 0.05) from the lower temperatures recorded in the cold-air drain at the wash site (black bar). |
TABLE 2. Extreme minimum temperatures °F (ground-level) at 9 experimental sites. Saguaro National Monument, 20 Dec. 1967 to 6 March 1968. Values shown are the lowest minimum recorded during during the interval since the last observation (3 Jan. observation is for the period beginning 20 Dec.) Data graphed in Figs. 9 and 10.
Plot site symbols: SW = west monument. SE = east monument. R = rocky habitat. F = flat habitat. N = north slope exposure. S = south slope exposure. O = open site (no cover). C = under tree crown (Cercidium microphyllum). U = unimpaired site adjacent to wash in cold air drain.
| Date (1968) | Plot |
Mean | ||||||||
| SEFC | SEFO | SEFUO | SERNO | SERSO | SWFC | SWFO | SWRNO | SWRSO | ||
| 3 January | 25 | 24 | 20 | 24 | 27 | 25 | 23 | 25 | 25 | 24.2 |
| 10 January | 25 | 23 | 20 | 27 | 31 | 26 | 23 | 27 | 30 | 25.8 |
| 17 January | 20 | 33 | 37 | 33 | 30 | 33 | 38 | 32.0 | ||
| 24 January | 22 | 28 | 33 | 28 | 26 | 30 | 35 | 28.9 | ||
| 31 January | 25 | 31 | 39 | 31 | 30 | 35 | 38 | 32.7 | ||
| 8 February | 24 | 30 | 27 | 29 | 28 | 33 | 35 | 29.4 | ||
| 15 February | 31 | 38 | 33 | 41 | 41 | 40 | 42 | 39.0 | ||
| 21 February | 40 | 39 | 35 | 38 | 42 | 41 | 39 | 40 | 42 | 39.6 |
| 6 March | 33 | 38 | 43 | 40 | 39 | 40 | 45 | 39.7 | ||
| Mean | 30.8 | 29.1 | 25.6 | 31.9 | 34.7 | 32.7 | 31.0 | 33.7 | 36.7 | |
On 3 January the stems of some injured plants exhibited white to yellow discoloration on the south side. By 10 January, severely damaged plants had a watery dark-green or black appearance, and desiccation of some individuals was evident. On 17 January, desiccation and collapse of six individuals had progressed to the extent that death could be confirmed. Similar symptoms preceded subsequently recorded deaths. Only one of the 86 recorded deaths was caused by insects.
As in the case reported above for 1968-69, survival at each of the nine 1967-68 winter study sites was associated with differences in winter minimum temperatures. Higher survival was associated with warmer sites as indicated by the recorded minimum temperatures (Tables 2, 4, 5; Figs. 10, 11, 12). The greatest survivorship, on rocky south-facing slopes, was associated with the highest mean minimum temperatures. Least survivorship was on north-facing slopes where minimum temperature means were 3.0°F (SW) and 3.8°F (SE) lower than those recorded on the opposing south-facing slopes. The survival and minimum temperature data suggest that the duration as well as the low extremes of temperature critically limit survival on north-facing slopes.
It is important to understand that saguaros do not freeze at 32°F—they supercool. Measured supercooling limits for saguaros from the Tucson region are —3.1°C to —12.4°C (mean —6.6°C 20.1°F); this range is from 26.4 to 9.9°F (see Lowe 1959). Assuming the same super-cooling capacity for the juvenile saguaro in these experiments, a difference of 3-4°F between slope exposures on coldest nights, as recorded, demands significantly different saguaro survival rates, as determined (Tables 2, 4, 5).
TABLE 3. Cumulative winter mortality and percent survival of known-age (30-42 month) juvenile saguaros (N = 180) at 9 sites in Saguaro National Monument, 15 December 1967 to 3 April 1968. Data graphed in Fig. 9.
| Date (1967-68) |
Mortality No. |
Survival | |
| No. | % | ||
| 15 December | 0 | 180 | 100.0 |
| 3 January | 0 | 180 | 100.0 |
| 10 January | 0 | 180 | 100.0 |
| 17 January | 6 | 174 | 96.7 |
| 24 January | 8 | 172 | 95.6 |
| 31 January | 20 | 160 | 88.9 |
| 8 February | 50 | 130 | 72.2 |
| 15 February | 62 | 118 | 65.6 |
| 21 February | 64 | 116 | 64.4 |
| 6 March | 75 | 105 | 58.3 |
| 13 March | 79 | 101 | 56.1 |
| 20 March | 82 | 98 | 54.4 |
| 27 March | 86 | 94 | 52.2 |
| 3 April | 86 | 94 | 52.2 |
TABLE 4. Summary of winter survival of known-age (30-42 month) juvenile saguaros in nine experimental plots at Saguaro National Monument east (SE) and west (SW), 15 December 1967 to 3 April 1968. N = 20 plants per plot: four 42-month-old plants (class of 1964), and sixteen 30-month-old plants (class of 1965). Data graphed in Fig. 11.
| Symbol | Plot Habitat |
Cover | Survivors | |||||
| Class of 1964 |
Class of 1965 |
Total | ||||||
| No. | % | No. | % | No. | % | |||
| SERNO | Rocks, N. Slope | Open | 2 | 50.0 | 1 | 6.2 | 3 | 15.0 |
| SERSO | Rocks, S. Slope | Open | 3 | 75.0 | 10a | 62.5 | 13 | 65.0 |
| SEFUO | Flats, Wash | Open | 4 | 100.0 | 3 | 18.8 | 7 | 35.0 |
| SEFO | Flats, Level | Open | 4 | 100.0 | 4 | 25.0 | 8 | 40.0 |
| SEFC | Flats, Level | Tree | 4 | 100.0 | 9 | 56.2 | 13 | 65.0 |
| SWRNO | Rocks, N. Slope | Open | 4 | 100.0 | 1 | 6.2 | 5 | 25.0 |
| SWRSO | Rocks, S. Slope | Open | 4 | 100.0 | 11 | 68.8 | 15 | 75.0 |
| SWFO | Flats, Level | Open | 4 | 100.0 | 11 | 68.8 | 15 | 75.0 |
| SWFC | Flats, Level | Tree | 4 | 100.0 | 11 | 68.8 | 15 | 75.0 |
| Total | 33 | 91.7 | 61 | 42.4 | 94 | 52.2 | ||
| N= | 36 | 144 | 180 | |||||
aMortality includes one insect-kill.
| ||||||||
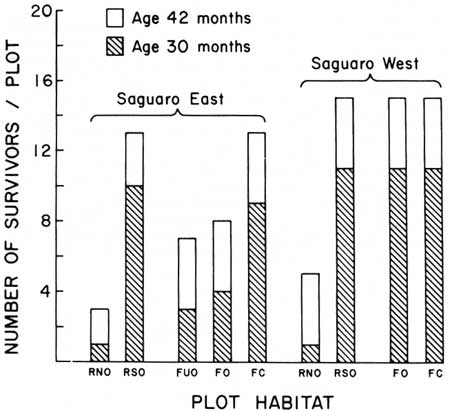
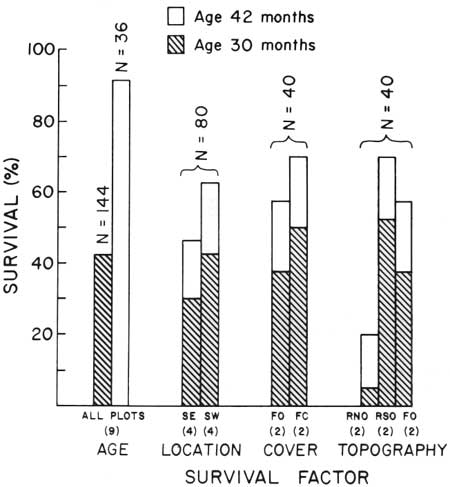
Differential winter mortality from freezing of 30- and 42-month-old juvenile saguaros occurred with differences (1) in age (plant size); (2) geographic location (east and west sections of the monument); (3) presence or absence of plant cover (paloverde tree, Cercidium microphyllum); and (4) topography (slope and direction of slope exposure. See Tables 4,5, and Fig. 12). The survival percentage (42.4%) for 30-month-old plants was less than half the survival percentage (91.7%) for 42-month-old plants. Survival at four east monument sites (46.2%) was lower than at four equivalent west monument sites (62.5%). Survivorship of plants in exposed plots was lower (57.5%) than survivorship in adjacent plots under paloverde tree (C. microphyllum) crown cover (70.0%). Highest survival (70.0%) was in south-facing slope habitats, the lowest survival, on north-facing slopes (2.0.0%). Survival in exposed flat habitats was intermediate (57.5%). In summary, during the period of observations (3.5 months), 48% of the saguaros in the sample died.
|
| Fig. 11. Survival of known-age juvenile (30 and 42 months) saguaros (N= 180) in nine experimental plots at Saguaro National Monument, east (SE) and west (SW), 15 December 1967 to 3 April 1968. Survival is shown as number of individuals per plot (N = 20) living on 3 April 1968. Shaded portion of each bar represents the number of surviving 30-month-old plants (class of 1965, N = 16). Unshaded portion represents the number of surviving 42-month-old plants (class of 1964, N = 4). Plot symbols: R = rocky habitat, N = north-facing slope. S = south-facing slope, F = flat habitat. C = under paloverde tree crown. O = open site (no cover plant). U = unpaired plot adjacent to wash in cold-air drain. Data in Table 4. Eighty-six of the 180 plants died between 10 January and 3 April: 85 deaths were caused by freezing, one individual was eaten by an insect (class of 1965, SE-RSO site). |
|
| Fig. 12. Differential survival of known-age juvenile saguaros at Saguaro National Monument. 15 December 1967 to 3 April 1968. Shaded portion of each bar represents surviving 30-month-old plants (class of 1965). ht. 10.61 mm (±0.16). Unshaded portion represents surviving 42-month-old plants (class of 1964), ht. 20.14 mm (±0.38). Plot symbols as in Table 2. Data in Table 5. Differential winter mortality from freezing occurred according to (1) differences in age (plant size); (2) geographic location (Saguaro Monument, east, and Saguaro Monument, west); (3) presence or absence of associated plant cover (paloverde tree, Cercidium microphyllum); and (4) topography (slope and direction of slope exposure). Number of plots in each group is shown in parenthesis. |
TABLE 5. Differential survival of known-age juvenile saguaros at nine sites. Saguaro National Monument, from 15 December 1967 to 3 April 1968. Mean height of 1964 plants (age 42 months) 20.14 mm (±0.38), height of 1965 plants (age 30 months) 10.61 mm (±0.16). Data graphed in Fig. 12. Plot site symbols as in Table 1.
| Sites | No. | Class of 1964 |
Class of 1965 |
Total | ||||||
| Survival |
Survival |
Survival | ||||||||
| N | No. | % | N | No. | % | N | No. | % | ||
| SWa | 4 | 16 | 16 | 100.0 | 64 | 34 | 53.1 | 80 | 50 | 62.5 |
| SEa | 4 | 16 | 13 | 81.2 | 64 | 24 | 37.5 | 80 | 37 | 46.2 |
| SEU | 1 | 4 | 4 | 100.0 | 16 | 3 | 18.8 | 20 | 7 | 35.0 |
| All plots | 9 | 36 | 33 | 91.7 | 144 | 61 | 42.4 | 180 | 94 | 52.2 |
| RNO | 2 | 8 | 6 | 75.0 | 32 | 2 | 6.2 | 40 | 8 | 20.0 |
| RSO | 2 | 8 | 7 | 87.5 | 32 | 21b | 65.6 | 40 | 28 | 70.0 |
| FO | 2 | 8 | 8 | 100.0 | 32 | 15 | 46.9 | 40 | 23 | 57.5 |
| SEU | 1 | 4 | 4 | 100.0 | 16 | 3 | 18.8 | 20 | 7 | 35.0 |
| FO | 2 | 8 | 8 | 100.0 | 32 | 15 | 46.9 | 40 | 23 | 57.5 |
| FC | 2 | 8 | 8 | 100.0 | 32 | 20 | 62.5 | 40 | 28 | 70.0 |
aPaired sites only.
bMortality includes one insect-kill. | ||||||||||

|
| Fig. 13. Flat habitat. Saguaro National Monument, Tucson Mountain Section. In this location, the saguaro occurs with paloverde (Cercidium microphyllum) and desert iron wood (Olnyea tesota) trees. Small saguaros are usually found beneath the crown of the predominant shrub (Ambrosia deltoidea = Franseria deltoidea). Photographed 16 April 1971. |
The 1971 Catastrophic Freeze
High winter mortality of seedlings and juvenile saguaros followed the period of critical subfreezing temperatures from 3 to 10 January 1971. Low minimum temperatures recorded at Tucson during the period ranged from 11°F on 7 January (Campbell Avenue Experimental Farm) to 19°F on 5 January (University of Arizona); data are from U.S. Weather Bureau (1971).

|
| Fig. 14. Rocky south-facing slope habitat. Saguaro National Monument. Tucson Mountain Section. This winter-warm habitat supports a vigorous stand of saguaros with abundant representation of juvenile and young adult plants. Photographed 16 April 1971. |
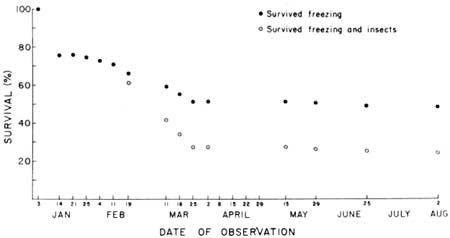
Winter survival of 30-month-old naturally growing juvenile plants in a 0.25-m2 plot located under the crown of a large Cercidium microphyllum at Saguaro Monument (east) is graphed in Fig. 15 (Table 6). Fifty-three (51.5%) of the 103 plants died as a result of the January freezing injuries and 25 plants (24.3%) were consumed by insects. Thirteen of the 25 remaining plants (52.1%) suffered obvious freeze-caused injuries that deformed their stems. Three severely freeze-deformed plants died during the hot months of May, June, and July. All insect-caused deaths occurred between 11 February and 25 March. A mature cutworm found feeding on young saguaros in an adjacent plot on 25 March was identified as Orthodes alfkeni Grote, a common native noctuid moth with general (nonspecific) plant-feeding habits.
|
| Fig. 15. Survival summary for 30-month-old saguaros (class of 1968) on flat habitat site (SEF, Fig. 7), Saguaro National Monument, east, 3 January to 2 August 1971. Open circles represent percent of original population (N = 103) surviving on the date of observation. Solid circles represent percent survival, insect-kill excluded, i.e., freeze survival. Data in Table 6. All insect-kill (25 plants consumed) occurred between 11 February and 25 March. All other deaths resulted from exposure to critical subfreezing temperatures occurring during the week beginning 3 January. Deaths recorded during May, June, and August (3 plants) were the ultimate result of January freeze damage that destroyed approximately 50% of the stem tissues of those plants. |
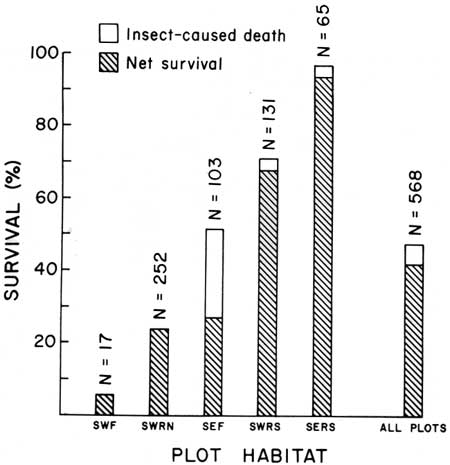
Winter survival of 30-month-old naturally growing saguaros under tree canopy and situated in flat, rocky north-facing and rocky south-facing slope habitats, is shown in Table 7 and Fig. 16. On 8 April, 57.9% (239 individuals) of the plants were dead from the January freeze-caused injury. Lowest mortality was on south-facing slopes. The highest mortality occurred on north-facing slopes and in flat habitats.
|
| Fig. 16. Winter survival of 30-month-old (class of 1968) saguaros in five habitats at Saguaro National Monument, 3 January to 8 April 1971. Total bar height represents percentage of plants that survived freeze-caused damage. Unshaded portion represents insect-caused deaths. Shaded portion represents net survival. Habitat designations are: SE = east monument. SW = west monument, F = flats. RN = rocky north-facing slope. RS = rocky south-facing slope. Data in Table 7. During critically cold winters, south-facing slopes offer the most suitable sites for survival of young plants. Relatively high mortality in 1971 was again associated with flat and north-facing slope habitats. |
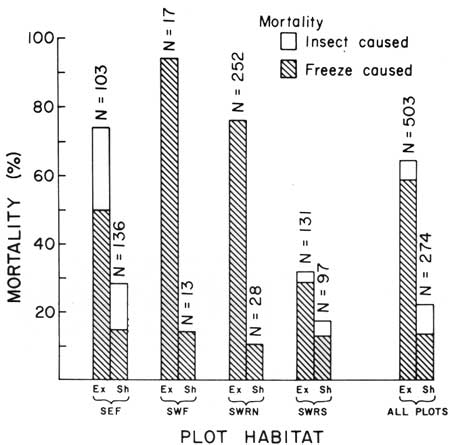
Mortality of 30-month-old juvenile saguaros in screen-shielded plots was substantially lower (22.3%) than mortality in paired, unshielded (mesh-covered) plots (64.9%, see Table 8 and Fig. 17). The nocturnal thermal shading effect of the two screening materials is markedly different, as reflected in the greater winter freeze-kill mortality under 0.5-inch mesh hardware-cloth than under window-screen. The more efficient window-screen reduced the nocturnal radiative heat loss to the zenith sky by 0.486 cal/min/cm2 compared to the hardware-cloth mesh. The difference is equivalent to 9°F (5°C) which is a significant and large nocturnal difference. Acting in a similar manner, low shrubs and tree cover provide a natural and relatively favorable thermal microenvironment for the survival of young saguaros during critically cold winter nights (see Lowe and Hinds 1972).
|
| Fig. 17. Differential mortality of young saguaros (age 30 mos.) in shielded (Sh) and exposed (Ex) plots at Saguaro National Monument. 3 January to 8 April 1971. Plot locations are: SE = east monument, SW = west monument, E = flats, RN = rocky north-facing slope. and RS = rocky south-facing slope. Shaded portion of each bar represents percent of freeze-caused deaths. Unshaded portion represents percent of insect-caused deaths occurring during the period (see Table 8). The moderating effect of low canopy during critically cold periods is evident in the substantially lower mortality within shielded plots. Freeze-caused deaths within window-screen shielded plots ranged from 53.8% to 83.6% (mean 76.4%) less than in the paired exposed (mesh-covered) plots. Acting in a similar manner, low-shrub canopy moderates the severity of freezing conditions providing a warmer thermal microenvironment favorable for the survival of young saguaros during critically cold winter nights. |
TABLE 6. Cumulative winter mortality and percent survival of 30-month-old juvenile saguaros (class of 1968) at Saguaro National Monument (east), flats. 3 January to 2 August 1971. Data graphed in Fig. 15 (Survivorship).
| Date (1971) |
Cumulative Mortality | |||||
| Insect |
Freezing |
Survival | ||||
| No. | % | No. | % | No. | % | |
| 3 January | 0 | 0.0 | 0 | 0.0 | 103 | 100.0 |
| 14 January | 0 | 0.0 | 25 | 24.3 | 78 | 75.7 |
| 21 January | 0 | 0.0 | 25 | 24.3 | 78 | 75.7 |
| 28 January | 0 | 0.0 | 26 | 25.2 | 77 | 74.8 |
| 4 February | 0 | 0.0 | 28 | 27.2 | 75 | 72.8 |
| 11 February | 0 | 0.0 | 30 | 29.1 | 73 | 70.9 |
| 19 February | 5 | 4.9 | 35 | 34.0 | 63 | 61.2 |
| 11 March | 18 | 17.5 | 42 | 40.8 | 43 | 41.7 |
| 18 March | 23 | 22.3 | 45 | 43.7 | 35 | 34.0 |
| 25 March | 25 | 24.3 | 50 | 48.5 | 28 | 27.2 |
| 2 April | 25 | 24.3 | 50 | 48.5 | 28 | 27.2 |
| 8 April | 25 | 24.3 | 50 | 48.5 | 28 | 27.2 |
| 15 April | 25 | 24.3 | 50 | 48.5 | 28 | 27.2 |
| 22 April | 25 | 24.3 | 50 | 48.5 | 28 | 27.2 |
| 29 April | 25 | 24.3 | 50 | 48.5 | 28 | 27.2 |
| 13 May | 25 | 24.3 | 50 | 48.5 | 28 | 27.2 |
| 29 May | 25 | 24.3 | 51 | 49.5 | 27 | 26.2 |
| 25 June | 25 | 24.3 | 52 | 50.5 | 26 | 25.2 |
| 2 August | 25 | 24.3 | 53 | 51.5 | 25 | 24.3 |
TABLE 7. Winter survival and mortality of 30-month-old saguaros (class of 1968) in five habitats at Saguaro National Monument. 3 January to 8 April 1971. Habitat designations are: SE = east monument, SW = west monument. F = flats. RN = rocky north-facing slope, and RS = rocky south-facing slope. Data graphed in Fig. 16 (Survivorship).
| Loc. | N | Live |
Freeze Kill |
Insect Kill | |||
| No. | % | No. | % | No. | % | ||
| SWF | 17 | 1 | 5.9 | 16 | 94.1 | 0 | 0 |
| SWRN | 252 | 60 | 23.8 | 192 | 76.2 | 0 | 0 |
| SEF | 103 | 28 | 27.2 | 50 | 48.5 | 25 | 24.3 |
| SWRS | 131 | 89 | 67.9 | 38 | 29.0 | 4 | 3.1 |
| SERS | 65 | 61 | 93.8 | 2 | 3.1 | 2 | 3.1 |
| Total | 568 | 239 | 42.1 | 298 | 52.5 | 31 | 5.5 |
TABLE 8. Winter mortality of 30-month-old (class of 1968) saguaros in shielded and unshielded (= exposed) plots at Saguaro National Monument. Data recorded are freeze-caused (Fr) and insect-caused (In) deaths in eight experimental plots. 3 January to 8 April 1971. At four sites, shielded plots covered with a 16-mesh (0.16 cm) window-screen exclosure were paired with exposed plots covered with a 0.5-inch mesh (1.3 cm) hardware-cloth exclosure, each located beneath the crown of a mature Cercidium microphyllum. Locations are: SE = east monument, SW = west monument, F = flats, RN = rocky north=facing slope, and RS = rocky south-facing slope. Data graphed in Fig. 17.
| Plot Loc. |
Under Cercidium Nurse-Plant | |||||||||||||
| Unshielded, Under Canopy |
Screen Shielded, Under Canopy | |||||||||||||
| N | Fr. | % | In. | % | Tot. | % | N | Fr. | % | In. | % | Tot. | % | |
| SEF | 103 | 51 | 49.5 | 25 | 24.3 | 76 | 73.8 | 136 | 20 | 14.7 | 19 | 14.0 | 39 | 28.7 |
| SWF | 17 | 16 | 94.1 | 0 | 0.0 | 16 | 94.1 | 13 | 2 | 15.4 | 0 | 0.0 | 2 | 15.4 |
| SWRN | 252 | 192 | 76.2 | 0 | 0.0 | 192 | 76.2 | 28 | 3 | 10.7 | 0 | 0.0 | 3 | 10.7 |
| SWRS | 131 | 38 | 29.0 | 4 | 3.0 | 42 | 32.0 | 97 | 13 | 13.4 | 4 | 4.1 | 17 | 17.5 |
| Total | 503 | 297 | 59.0 | 29 | 5.8 | 326 | 64.8 | 274 | 38 | 13.9 | 23 | 8.4 | 61 | 22.3 |
Age, Height, and Mortality
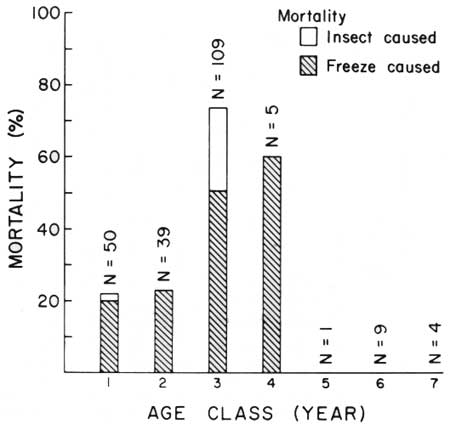
Age-class winter mortality of young saguaros (seedlings and globose juveniles, 1st through 7th year of life) in flat habitat is shown in Table 9 and Fig. 18. The data clearly indicate greater freeze-susceptibility in this microenvironment during the first 1-4 years of life. There was zero mortality of 5th year and older plants. Vulnerability of young plants to destruction by freezing varies with age (i.e., size) of the young plant.
|
| Fig. 18. Age-class winter mortality of known-age (1st to 7th year) seedling and juvenile saguaros in flat habitat at Saguaro National Monument, east. 3 January to 8 April 1971. Shaded portion of bar represents percent of freeze-caused mortality; unshaded portion represents insect-caused mortality. Vulnerability of young plants to destruction by freezing varies with the age, i.e. size of the young plant. The data clearly indicate greater freeze susceptibility in this microenvironment during the first 1-4 years of life. There was zero mortality during and after 5 years. Data in Table 9. |
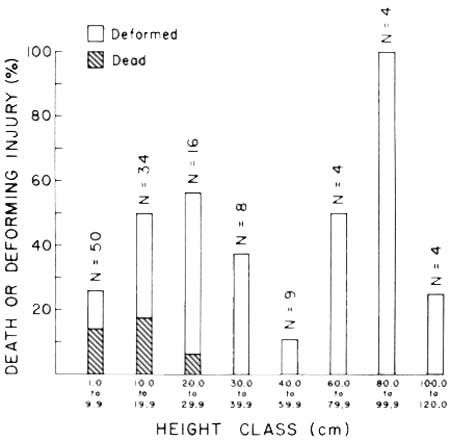
Height-class freeze-caused mortality and deforming injury of juvenile saguaros less than 120 cm in height in flat habitats is shown in Table 10 and Fig. 19. Observable deforming injuries to stem tissues occurred in all height-classes. Only plants less than 30 cm in height were completely destroyed by freezing (Fig. 20). Resistance of juvenile saguaros to death from freezing increases with age-related changes in the size and form of the plants (Figs. 1, 4, 5, 6).
|
| Fig. 19. January 1971 freeze-caused mortality and deforming injury of juvenile saguaros (N = 129) in flat habitats at Saguaro National Monument to 9 August 1971. Shaded portion of bars represents percentage of deaths in each class. Unshaded portion represents per cent of class with observable deforming injuries. Plants in all size-classes sustained deforming injuries. Only plants less that 30 cm in height were completely destroyed by freezing. Data in Table 10. |
Population Structure and the Climatic Record
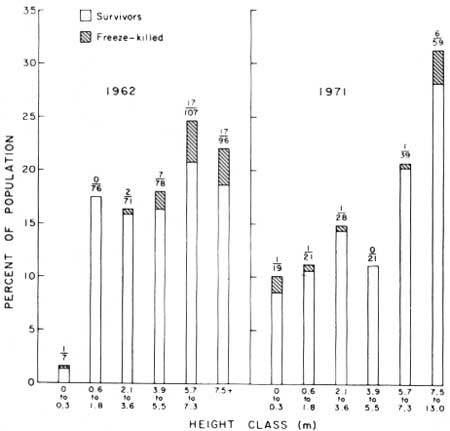
Height-class distribution of freeze-caused saguaro deaths in natural populations at Saguaro National Monument, resulting from recent catastrophic freezes, in January 1962 and January 1971, are shown in Tables 11, 12, and Fig. 21. In both samples, the highest rates of mortality were in the smallest and largest plant size-classes (Figs. 20, 22, 23). The combined freeze-caused mortality data for both years show that freezing killed 15.4% of the plants less than 0.45 m in height, 2.0% of the plants 0.46-3.80 m in height, and 12.0% of all plants over 3.80 m in height.

|
| Fig. 20. Freeze-killed juvenile saguaro (ht. 6.5 cm) at Saguaro National Monument, west section. Twelve days after the onset of the 1971 catastrophic freeze the blackish collapsed young plant has begun to desiccate. Photographed 15 January 1971. |
|
| Fig. 21. Freeze-caused mortality in saguaro populations at Saguaro National Monument. Distribution by height-class of saguaro deaths from freezing during January 1962 and January 1971. The 1962 data are for both east and west monument sections from Niering et al. 1963 (Table 11). The 1971 data are from west monument section only (Table 12). Height of bar indicates percent of total population occurring in each height-class; shaded portion indicates percent of class killed by freezing. Above each bar, the number of freeze-killed plants is shown over the total number of plants in that class. The greatest mortality occurred in the smallest and the largest age-classes in both 1962 and 1971. |
It is clear from these data that catastrophic freezing selectively structures saguaro populations, removing the smallest (youngest) and the largest (oldest) plants, leaving a high percentage of the large juvenile and unbranched young adult plants with heights from 0.46 to 3.80 m (1.5-12.5 ft, Fig. 7). The size-class (= age) structure of the saguaro population at Saguaro National Monument (east) flats extant in 1942 (Table 13; Fig. 24) is that of a declining population (Gill 1951; Gill and Lightle 1942, 1946; Mielke 1944). The estimated age-ranges assigned to the size-classes represented in that population permits deterioration of the approximate year of their germination. Thus the age- and height-range of the surviving plants in each class can be estimated for a previous year. The estimated heights of 1942 saguaros living in 1913 at Saguaro National Monument (east) are given in Table 13. These data are of particular interest for they clearly show the selective structuring of the population that results from catastrophic freeze-kill as revealed in the climatic record and in the data on freeze-caused mortality.
TABLE 9. Winter mortality of known-age seedlings and juvenile saguaros in flat habitat at Saguaro National Monument (east), 3 January to 8 April 1971. Data graphed in Fig. 18.
| Age Class (Yr.) |
Yr. Germ. |
N | Deaths | |||||
| Survivors |
Freezing |
Insects | ||||||
| No. | % | No. | % | No. | % | |||
| 1 | 1970 | 50 | 39 | 78.0 | 10 | 20.0 | 1 | 2.0 |
| 2 | 1969 | 39 | 29 | 74.4 | 9 | 23.1 | 0 | 0.0 |
| 3 | 1968 | 109 | 29 | 26.6 | 55 | 50.5 | 25 | 22.9 |
| 4 | 1967 | 5 | 2 | 40.0 | 3 | 60.0 | 0 | 0.0 |
| 5 | 1966 | 1 | 1 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| 6 | 1965 | 9 | 9 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| 7 | 1964 | 4 | 4 | 100.0 | 0 | 0.0 | 0 | 0.0 |
| Total | 217 | 113 | 52.1 | 77 | 35.5 | 26 | 12.0 | |
TABLE 10. Mortality and deforming injury to 9 August 1971 of juvenile saguaros 1-120 cm height (N = 129) at Saguaro National Monument caused by freezing conditions during January 1971. Deformed plants are individuals with observable dead stem tissues. Data graphed in Fig. 19.
| Height-class (cm) |
N | No Deformity |
Critical Injury | ||||||
| Killed |
Deformed |
Total | |||||||
| No. | % | No. | % | No. | % | No. | % | ||
| 1.0-9.9 | 50 | 37 | 74.0 | 7 | 14.0 | 6 | 12.0 | 13 | 26.0 |
| 10.0-19.9 | 34 | 17 | 50.0 | 6 | 17.6 | 11 | 32.4 | 17 | 50.0 |
| 20.0-29.9 | 16 | 7 | 43.8 | 1 | 6.2 | 8 | 50.0 | 9 | 56.2 |
| 30.0-39.9 | 8 | 5 | 62.5 | 0 | 0.0 | 3 | 37.5 | 3 | 37.5 |
| 40.0-59.9 | 9 | 8 | 88.9 | 0 | 0.0 | 1 | 11.1 | 1 | 11.1 |
| 60.0-79.9 | 4 | 2 | 50.0 | 0 | 0.0 | 2 | 50.0 | 2 | 50.0 |
| 80.0-99.9 | 4 | 0 | 0.0 | 0 | 0.0 | 4 | 100.0 | 4 | 100.0 |
| 100.0-119.9 | 4 | 3 | 75.0 | 0 | 0.0 | 1 | 25.0 | 1 | 25.0 |
| Total | 129 | 79 | 14 | 36 | 50 | ||||
| Percent | 100.0 | 61.2 | 10.8 | 27.9 | 38.8 | ||||
TABLE 11. Distribution by height-class of January 1962 freeze-caused saguaro mortality in three habitats at Saguaro National Monument. Data from ten 0.1ha samples in each habitat (Niering et al. 1963). Data graphed in Fig. 21.
| East Monument |
|||||||||||||
| Height-class |
Slopes |
Flatsa |
West Monument Flatsa |
All Monument | |||||||||
| Feet | Meters | N | Fr. | % Fr. | N | Fr. | % Fr. | N | Fr. | % Fr. | N | Fr. | % Fr. |
| To 1 | To 0.3 | 5 | 0 | 0.0 | 0 | 0 | 0.0 | 2 | 1 | 50.0 | 7 | 1 | 14.3 |
| 2-6 | 0.6-1.8 | 10 | 0 | 0.0 | 6 | 0 | 0.0 | 60 | 0 | 0.0 | 76 | 0 | 0.0 |
| 7-12 | 2.1-3.6 | 16 | 2 | 12.5 | 7 | 0 | 0.0 | 48 | 0 | 0.0 | 71 | 2 | 2.8 |
| 13-18 | 3.9-5.5 | 17 | 4 | 23.5 | 21 | 3 | 14.3 | 40 | 0 | 0.0 | 78 | 7 | 9.0 |
| 19-24 | 5.7-7.3 | 23 | 8 | 34.8 | 41 | 8 | 19.5 | 43 | 1 | 2.3 | 107 | 17 | 15.9 |
| Over 24 | Over 7.5 | 15 | 3 | 20.0 | 55 | 12 | 21.8 | 26 | 2 | 7.7 | 96 | 17 | 17.7 |
| Total | 86 | 17 | 19.8 | 130 | 23 | 17.7 | 219 | 4 | 1.8 | 435 | 44 | 10.1 | |
aSynonymous with "bajada" as used in the original publication. | |||||||||||||
TABLE 12. Distribution by height-class of 1971 freeze-caused saguaro mortality in flat habitat at Saguaro National Monument (west). Height and condition of all saguaros (N = 189) in a 2-ha plot (100 x 200 m). Data obtained January to November 1971. Graphed in Fig. 21.
| Dead |
||||||||
| Height-Class |
Live | Wind |
Freezing |
Total | ||||
| Feet | Meters | No. | No. | % | No. | % | No. | % |
| To 1 | To .45 | 16 | 0 | 0.0 | 3 | 15.8 | 19 | 10.1 |
| 2-6 | .46-1.97 | 20 | 0 | 0.0 | 1 | 4.8 | 21 | 11.1 |
| 7-12 | 1.98-3.80 | 27 | 0 | 0.0 | 1 | 3.6 | 28 | 14.8 |
| 13-18 | 3.81-5.63 | 21 | 0 | 0.0 | 0 | 0.0 | 21 | 11.1 |
| 19-24 | 5.64-7.46 | 38 | 0 | 0.0 | 1 | 2.6 | 39 | 20.6 |
| 25-43 | 7.47-13.00 | 52 | 1 | 1.7 | 6 | 10.2 | 59 | 31.2 |
| Broken | 2 | 0 | 0.0 | 0 | 0.0 | 2 | 1.1 | |
| Total | 176 | 1 | 0.5 | 12 | 6.35 | 189 | 100.0 | |

|
| Fig. 22. Freeze-killed decomposing saguaro in "Cactus Forest" area, Saguaro National Monument (east). Drooping arms are a common indicator of damaging freeze-caused injury during earlier years. Leaning and slender top, drooping arms, and scarred stem on senescent saguaro behind, also indicate old freeze-caused damage, that is common in this population. Photographed 15 April 1971. |

|
| Fig. 23. Freeze-caused decapitation of saguaros at Saguaro National Monument (east). Collapsed, freeze-killed tissues at the site of woodpecker holes, as in the stem and arm of the right-hand plant, have caused decapitation of the saguaro on the left. Photographed 28 May 1971. |
TABLE 13. Age-survivorship relationship of saguaros at Saguaro National Monument (east), based on height-class composition of that population in January 1942. Data on living saguaros (N = 12,698) in a 1-mile2 (2.59 km2) plot (Sect. 17. flat habitat) from Gill and Lightle 1942. Data graphed in Fig. 24.
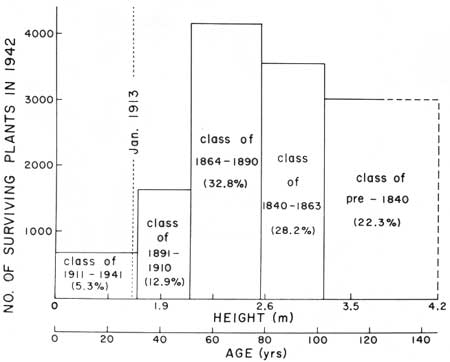
The average number of plants from each year class surviving to 1942 (surv./yr.) is obtained by dividing the number of plants in each class by the year interval for that class. January 1913 age-height relationships for plants of each class are shown in the last two columns.
| 1942 |
1913 | ||||||
| Height-Class |
Agea class (yrs.) |
Class int. (yrs.) |
Living Saguaros |
Age (yrs.) |
Ht. (m) | ||
| Feet | Meters | No. | Surv./yr. | ||||
| 0—6 | 0.0—1.9 | 0—31 | 31 | 678 | 21.87 | 0—2 | 0.00—0.01 |
| 7—12 | 2.0—3.8 | 32—51 | 20 | 1638 | 81.90 | 3—22 | 0.01—0.49 |
| 13—18 | 3.9—5.6 | 52—78 | 27 | 4163 | 154.18 | 23—49 | 0.55—3.7 |
| 19—24 | 5.7—7.5 | 79—102 | 24 | 3581 | 149.21 | 50—73 | 3.80—5.0 |
| 25+ | 7.6+ | 103+ | 2834 | 74+ | 5.1+ | ||
| Unclassified (broken stems) | 74 | ||||||
aAge estimates based on saguaro growth curves for young
plants less than 1 m high from on-going investigations by authors; see
also Shreve (1910) and Hastings and Alcorn 1961.
| |||||||
|
| Fig. 24. Number and age of saguaros surviving to January 1942 at Saguaro National Monument, east (flats). Population data for 12,698 saguaros, from Gill and Lightle 1942, see Table 13. The disproportionately small representation (18.2%) of plants surviving from the two youngest age-classes (plants 1-51 years old) is clearly indicative of a declining local population. The obviously unbalanced age structure of the population results from recurring catastrophic freezes acting differentially (1) upon small, highly vulnerable seedlings and juvenile plants, and (2) on the relatively freeze-resistant larger juvenile and young adult plants between approximately 1.0-3.5 m in height. In January 1913, when a record low temperature of 6°F (—16.5°C) was recorded in Tucson, plants of the 1891-1910 year-classes would have attained at that time maximum heights of less than 0.5 m (age 3-22 years); plants of the 1864-90 year-classes would have grown to heights of 0.5 to 3.7 m (age 23-49 years). The large proportion (32.8%) of surviving plants from the latter class (1864-90) and from the two older classes (50.5%) strongly suggests that catastrophic freezes were not a characteristic of the environment in which those particular plants originated, i.e. during the period from 50 to 100 years or more prior to 1890. Since that date, however, the semi-regular occurrence of catastrophic freezes is a matter of record (see Fig. 25), and the disproportionately small representation of juvenile and young adult saguaros in this population is clearly attributable to the repeated occurrence of these devastating winter climatic events. |
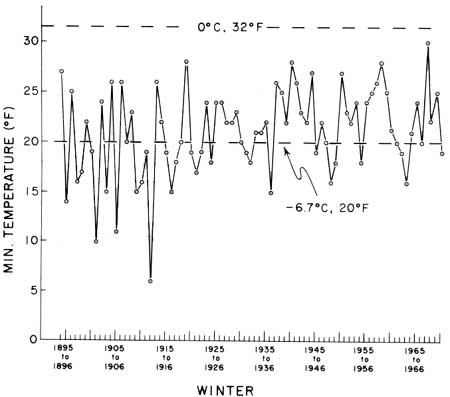
The climatic summary of minimum winter temperatures at Tucson, Arizona (U.S. Weather Bureau 1894-1971), graphed in Fig. 25, shows that the lowest temperature ever recorded in Tucson occurred 13 January 1913, when a minimum temperature of 6°F (—16.5°C) was observed. Minimum temperatures of 20°F or lower were recorded during 38 of the 77 consecutive winters spanned by this record. Fully 17 of these events occurred during the 18 years from 1894 to 1913, when temperature readings of 20°F or lower were recorded one or more times during 13 winters in that period. During the 58 years since 1913, the short 8-year period between January 1937 and December 1945 was the longest interval between occurrences of such critical subfreezing temperatures.
|
| Fig. 25. Climatic summary, minimum winter temperatures, Tucson, Arizona. Temperatures (°F) are the low minimum recorded during the months of December through February of each winter, 1894-95 through 1970-71, at the University of Arizona Station. Data from Climatological Data Summaries, Arizona, Weather Bureau, U.S. Department of Agriculture and U.S. Department of Commerce, since December 1894. Minimum temperatures of 20°F or lower were recorded during 38 (approximately one-half) of the 77 consecutive winters spanned by this record. Seventeen of those events occurred during the 18 years from 1894 to 1913; temperature readings 20°F or lower were recorded one or more times during 13 winters in that period. During the 58 years since January 1913, the 8-year period between January 1937 and December 1945 was the longest interval between the occurrence of such critical subfreezing temperatures. |
The disproportionately small representation (18.2%; Table 13; Fig. 24) of plants surviving from the two youngest age-classes (plants 1-51 years old) is clearly indicative of a declining population. At Saguaro National Monument (east), the obviously unbalanced age structure of the population results from recurring catastrophic freezes acting differentially upon (1) highly vulnerable seedlings and small juvenile plants (Figs. 3, 4, 5, 6), and (2) the relatively freeze-resistant larger juvenile and young adult plants between approximately 1.0 and 3.5 m in height (Fig. 7). We calculate that in the deep freeze of January 1913, plants of the 1891 to 1910 year-classes would have attained at that time the vulnerable maximum heights of juveniles less than 0.5 m (age 3-22 years)—plants of the 1864-90 year-classes would have grown to heights of 0.5-3.7 m (age 23-49 years).
The large proportion (32.8%) of surviving plants from the latter class (1864-90) and from the two older classes (50.5%) strongly suggests that catastrophic freezes were not a characteristic of the environment in which those particular plants originated, i.e., during the period from 50 to 100 years or more prior to 1890. Since that date, moreover, the semi-regular occurrence of catastrophic freezes is a matter of record and the disproportionately small representation of juvenile and young adult saguaros in this monument population is clearly assignable to the repeated occurrence of these devastating climatic events.
SUMMARY AND CONCLUSIONS
Saguaro populations at the eastern and northern limits of the species range in northern Sonora and in Arizona are subject to recurring extremes of subfreezing winter temperatures. These events, acting in a catastrophic manner, are the primary control on the limits, local distribution, and dynamics of saguaro populations in southeastern (and northern) Arizona.
In short, in this area of their geographic range, saguaros frequently freeze to death. Accordingly, in this northern portion of the plant's range, saguaro populations at the eastern, northern, and extreme elevational limits of the species distribution are limited to specific topographic situations (microhabitats) that effectively moderate the intensity and duration of critical winter minimum temperatures, i.e., south-facing slopes, rocky footslopes, and the upper portions of adjoining valleys. These are winter-warm microenvironments. The saguaro is absent or rare on colder, north-facing slopes and areas of cold air drainage and accumulation.
Within these northern populations, differential mortality of young saguaros occurs in dissimilar microhabitats, and is attributable to differing physical characteristics of the microenvironments there. This importantly involves the protective stratification of associated plants.
Under freezing conditions, survival of the smallest saguaros is dependent upon the physical characteristics of the microhabitat—specifically, spatial relationships with other plants, rock and other objects that mitigate the thermal environment of the plant during and immediately following the period of freezing conditions. Population density in the long run is limited by the availability of winter-favorable microhabitats that permit survival of young plants through critical extremes of subfreezing temperatures.
The percent mortality of each age-class of saguaros and the range of plant sizes affected by a particular freeze will depend upon the severity of freezing conditions, the intensity and duration of subfreezing temperatures, and other weather conditions preceding, during, and immediately following the freeze.
Vulnerability of saguaros to death from freezing is correlated with plant size and form (age). Catastrophic freezing selectively removes the youngest and the oldest members from the plant population and favors the survival of intermediate age-classes.
Accordingly, the continuing decline of saguaro populations at Saguaro National Monument and the present size-class structure of these populations is primarily the result of recurring catastrophic freezes acting differentially upon (1) seedlings and small juvenile plants; (2) larger juvenile and young adult plants; and (3) large adult plants.
Saguaro populations fluctuate in direct response to recurring catastrophic freezes in a characteristic and predictable manner, each freeze producing an abrupt reduction in the population, followed during the period of remission by gradual regrowth toward the original population level. Under such recurring stress, the ultimate trend of the population is determined by the relative severity of successive catastrophic freezes and the relative interval between successive freezes. If no change occurs either in the length of the interval between freezes or in the severity of successive freezes, the population could fluctuate about a more or less stable mean value and exhibit a stable age distribution. If the intervals between freezes become progressively longer or freeze severity progressively decreases, the population would increase until another factor becomes limiting. If the intervals become progressively shorter or the severity increases, the population will be unable to reach a stable age distribution and will eventually decline to extinction in the affected portion of its range.
The trend and ultimate fate of natural saguaro populations in northeastern Sonora and southeastern Arizona, including Saguaro National Monument, depend upon the future winter climate of the region—specifically, upon the frequency and intensity of catastrophic freezes. Our ability to predict the ultimate fate of some of these populations is contingent upon, and limited by, the ability to predict the occurrence of such events over a long period of time.
MANAGEMENT RECOMMENDATIONS
Under the authority of the Antiquities Act of 8 June 1906, Saguaro National Monument was set aside because of its scientific interest—specifically for the intrinsic interest of the natural vegetation therein.
The primary significance of Saguaro National Monument, therefore, lies in the natural associations of the vegetation found within its boundaries. The monument contains the last remaining example of an essentially undisturbed continuum of natural warm-desert to mountain-forest biotic associations in the southwestern United States. The singular rarity of this resource clearly indicates the importance of maintaining the integrity of the natural associations and relationships within Saguaro National Monument. It is upon consideration of the legislated purpose, the intrinsic natural significance of the area, and the scientific and cultural values of the resource that the following management recommendations are based:
Exclude developments and associated intensive use from responsive, uncommon, or rare habitats and natural communities. All use causes some deterioration of saguaro and other habitats. The question of what constitutes an acceptable level of destruction must accompany every decision to accommodate such use of the monument.
Eliminate picnic areas from saguaro habitats. Soil compaction, wood-gathering, and vandalism associated with these developments contribute to the degeneration of the site and adjacent habitat, ultimately leading to the death of existing saguaros and precluding the establishment and survival of young plants.
Limit further developments within saguaro habitats to those that will not attract destructive use and cannot be located elsewhere.
Develop management programs to provide more effective control of activities that are directly destructive to natural populations, communities, and habitats.
Control uses that are destructive to saguaro habitat such as off-pavement vehicle parking and off-trail foot and horse travel. Where necessary provide and direct the use of appropriate facilities for such activities.
Intensify management programs to control increasing vandalism and removal of saguaros. Old plants destroyed will not be replaced in a human lifetime. Young plants destroyed are those few that have survived the many hazards of the first critical years of life.
Eliminate cattle grazing. Continuing consumptive use by these exotic animals has a devastating impact upon the biotic as well as the esthetic environment. Grazing intensifies detrimental actions of natural environmental factors.
Continue research designed to obtain basic information on population and community dynamics and institute new programs to facilitate related studies.
Continue on-going saguaro population studies and institute additional studies on related communities. The response of saguaro populations and the associated biotic communities—past, present, and future—provides a valuable measure of climatic change and the resulting effects.
Institute additional studies to inventory and estimate the status and trend of saguaro populations and other key species.
Establish weather stations and maintain accurate and consistent weather records using standard calibrated instruments and recognized procedures. Lack of reliable on-site climatic data has been a major handicap in efforts to relate environmental factors to saguaro population changes.
Map and identify all transplanted saguaros surviving from previous research activities. The absence of such identification precludes the obtaining of accurate information on natural survival at those locations.
Encourage and facilitate nondestructive independent scientific research activities appropriate to the purpose and significance of the area.
Incorporate research findings into the interpretive program, stressing evolution and physical environment in relation to populations and communities.
Allow continuation of natural regenerative processes in saguaro habitats from which adverse use has been eliminated. Avoid interference with these processes by avoiding the introduction of horticultural and other programs that will unbalance on-going natural recovery of deteriorated habitats.
LITERATURE CITED
BENSON, L. 1969. The cacti of Arizona. Univ. of Arizona Press, Tucson.
DESPAIN, D. G., L. C. BLISS, and J. S. BOYER. 1970. Carbon dioxide exchange in saguaro seedlings. Ecology 51(5):9 12-914.
EARLE, W. H. 1963. Cacti of the southwest. Desert Botanical Garden of Arizona Sci. Bull. 4:1-112.
GILL, L. S. 1951. Mortality in the giant cactus at Saguaro National Monument 1941-1950. Saguaro National Monument Headquarters (Tucson, Arizona), Official Report; 1-5, 2 Tables, 1 Fig.
______, and P. C. LIGHTLE 1942. Cactus disease investigation. Saguaro National Monument Headquarters (Tucson, Arizona), Official Report 1-40, 9 Tables, 15 Figs.
______. 1946. Analysis of mortality in saguaro cactus. Saguaro National Monument Headquarters (Tucson, Arizona), Official Report; 1-4, 11 Tables.
HARPER, J. L. 1967. A Darwinian approach to plant ecology. J. Anim. Ecol. 36(3):495-518.
HASTINGS, J. R., and S. M. ALCORN. 1961. Physical determinations of growth and age in the giant cactus. J. Ariz. Acad. Sci. 2(1):32-39.
______, and R. M. TURNER. 1965. The changing mile: an ecological study of vegetation change with time in the lower mile of an arid and semi-arid region. Univ. of Arizona Press, Tucson.
LOWE, C. H. 1959. Contemporary biota of the Sonoran Desert Problems. Pages 54-74 in Univ. Arizona, Arid Lands Colloquia, 1958-59.
______. 1966. Life and death of the saguaro in Arizona. Cactus Capital Chatter 1(8):2-3.
______, and D. S. HINDS. 1972. Effect of paloverde (Cercidium) trees on the radiation flux at ground level in the Sonoran Desert in winter. Ecology 52(5):916-922.
McDONOUGH, W. T. 1964. Germination responses of Carnegiea gigantea and Lemaireocereus thurberi. Ecology 45(1):155-159.
MIELKE J. L. 1944. Summary of results of control experiments on saguaro disease. Saguaro National Monument. Saguaro National Monument Headquarters (Tucson, Arizona), Official Report: 1-4.
NIERING, W. A., R. H. WHITTAKER, and C. H. LOWE. 1963. The saguaro: A population in relation to environment. Science 142(3588):15-23.
SHREVE, E. 1910. The rate of establishment of the giant cactus. Plant World 13(10):235-240.
______. 1911. The influence of low temperatures on the distribution of the giant cactus. Plant World 14(6): 136-146.
______. 1951. Vegetation of the Sonoran Desert. Carnegie Inst. Washington Publ. 591:1-192.
SNYDER, E. E., and D. J. WEBER 1966. Causative factors of cristation in the Cactaceae. Cactus and Succulent J. 38(1):27-32.
SOULE O. H., and C. H. LOWE. 1970. Osmotic characteristics of tissue fluids in the saguaro giant cactus (Cereus giganteus) Ann. M. Bot. Gard. 57(3):265-351.
STEELINK, C., E. RISER, and M. J. ONORE. 1968. Carbohydrate constituents of healthy and wound tissue in the saguaro cactus. Phytochemistry 7:1673-1677.
______, M. YEUNG, and R. L. CALDWELL. 1967. Phenolic constituents of healthy and wound tissues in the giant cactus (Carnegiea gigantea). Phytochemistry 6:1435-1440.
STEENBERGH, W. E. 1970. Rejection of bacterial rot by adult saguaro cacti (Cereus giganteus). J. Ariz. Acad. Sci. 6(1):78-81.
______. 1972. Lightning-caused destruction in a desert plant community. Southwest. Nat. 16(3/4):419-429.
______, and C. H. LOWE. 1969. Critical factors during the first year of life of the saguaro (Cereus giganteus) at Saguaro National Monument, Arizona. Ecology 50(5):825-834.
THORNBER, J. J. 1911. Plant acclimatization in southern Arizona. Plant World 14:15-23.
THORNBER, J. J. 1916. Introduction. Pages 119-122 in J. C. T. Uphof, Cold resistance in spineless cacti. Univ. Ariz. Exp. Stn. Bull. 79.
TURNAGE, W. V., and A. L. HINCKLEY. 1938. Freezing weather in relation to plant distribution in the Sonoran Desert. Ecol. Monogr. 8:529-550.
TURNER, R. M., S. M. ALCORN, and G. OLIN. 1969. Mortality of transplanted saguaro seedlings. Ecology 50(5):835-844.
______, S. M. ALCORN, G. OLIN, and J. A. BOOTH. 1966. The influence of shade, soil and Water on saguaro seedling establishment. Bot. Gaz. 127(2-3):95-102.
U.S. WEATHER BUREAU. 1894-1940. Climatological Data Summaries, Arizona.
U.S. WEATHER BUREAU. 1940-1971. Climatological Data Summaries, Arizona.
Acknowledgments
Superintendent Harold R. Jones and the members of the Saguaro National Monument staff have aided the conduct of this study in many ways. We are grateful to Robert M. Linn and members of the National Park Service's Office of the Chief Scientist for their support of the saguaro ecology investigations reported here and elsewhere. We are indebted to E. Annette Halpern, David S. Hinds, Richard D. Krizman, John S. Phelps, Oscar O. Soule, and Thomas A. Wiewandt for valuable assistance in this study. We also thank Floyd G. Werner for insect identification. Lupe P. Hendrickson deserves special thanks for devoted assistance in preparation of the manuscript.
The research was supported by National Park Service Research Contract No. 14-10-0333-1303, the National Park Service's Office of the Chief Scientist, and Saguaro National Monument.
| <<< Previous | <<< Contents >>> | Next >>> |
chap5.htm
Last Updated: 1-Apr-2005