
|
Geological Survey Bulletin 395
Radioactivity of the Thermal Waters of Yellowstone National Park |
APPARATUS.
DESCRIPTION OF ELECTROSCOPES.
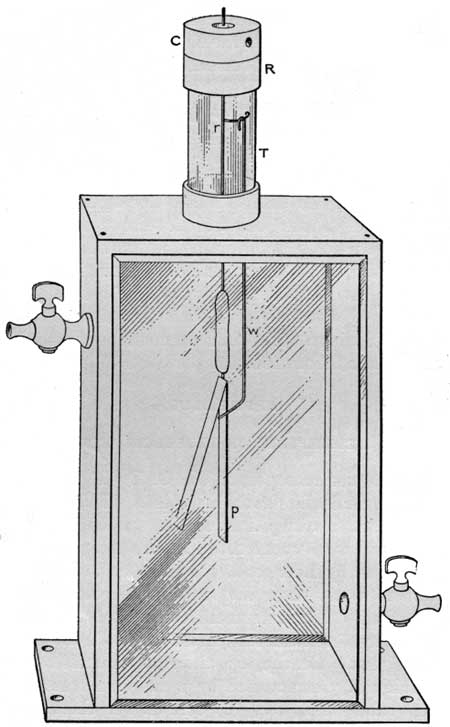
Three air-tight electroscopes of the C. T. R. Wilson type were employed in the field and laboratory tests. Two of these we had used in a previous investigation on the radioactivity of waters.a These instruments were constructed in the main according to the specifications given by Boltwood.b Figure 1 shows one of the electroscopes but does not show the reading microscope for observing the fall of the leaf. The instrument consists of a rectangular brass frame 15 by 10 by 6 centimeters, fitted on the front and back with pieces of plate glass. On the top, the frame carries a short piece of wide glass tubing (T) surmounted by a threaded brass ring (R), into which a removable brass cap (C) screws. A short piece of glass tubing passes through a hole in the cap and a brass rod (r) is cemented in the glass tube by means of sulphur or wax. The lower end of the brass rod extends into a rod of sulphur, which serves as the insulating material to support the leaf system. The plate (p) to which the leaf is fastened is embedded for a short distance in the other end of the sulphur rod. A special feature of the electroscope is the device for charging it.c A piece of soft iron wire (w) is suspended from a hook on the brass rod (r) which projects out through the screw cap. The wire extends just below the sulphur insulator and terminates in a right-angle bend. In its normal position the wire does not touch the plate carrying the leaf, but by bringing a magnet near the wire, it can be deflected so as to make metallic contact between the rod (r) and the plate (p). All of the brass joints are soldered and the other joints are made air-tight by means of hot wax, except that between the brass ring and cap, where a rubber washer excludes the air. The electroscope is provided with two good brass stopcocks.
aJour. Phys. chem., vol. 9, 1905, p. 321.
b Am. Jour. Sci., 4th ser., vol. 18, 1904, p. 97.
cThe same device was used by Strutt, Phil. Mag., 6th ser., vol. 5, 1903, p. 680; and by Boltwood, loc. cit.
|
| FIGURE 1.—Electroscope. |
Aluminum leaf was used in the instruments. It was readily and firmly fastened to the carrying blade by moistening the upper portion of the blade and then pressing it firmly against the leaf. The sulphur rod and the aluminum leaf successfully withstood the transportation by rail and in the field. In two of the electroscopes the original leaf served throughout the entire series of field experiments. The volume of the electroscopes was, respectively, 490, 590, and 600 cubic centimeters. A rod of vulcanite about 20 centimeters long was used for charging. After metallic connection between the leaf system and the projecting rod had been made, the charged vulcanite was touched to the brass rod, and by gentle rubbing, the desired potential on the leaf system was produced. After withdrawing the vulcanite the rod and cap were earthed for an instant.
Each electroscope was fastened securely to a wooden base. The fall of the leaf was observed by means of a microscope firmly supported by a brass holder which was likewise screwed to the wooden base. The glass scale in the eyepiece was divided into ten divisions and each of these was again divided into tenths. The diameter of the field corresponding to the hundred small divisions of the scale was approximately 5.2 millimeters for each of the microscopes. The rate of fall of the leaf was generally obtained by noting the time required for the leaf to fall 60 small divisions between the same two points of the scale. The time interval was measured by means of a stop watch recording fifths of a second. To secure uniform and ready adjustment of the instruments for the field tests a circular level was attached to the base. Suitable carrying cases were constructed for the electroscopes to facilitate their safe transportation in conducting the outdoor experiments.
STANDARDIZING THE ELECTROSCOPES.
To make quantitative activity measurements by means of electroscopes, each instrument must be standardized. The method of standardizing employed was the one first proposed by Boltwood,a which we had adopted in our earlier electroscopic determinations of radium emanation in waters.b Strutt has followed a similar procedure in his extensive investigations on the radium content of rocks and spring deposits.c The method is based upon the well-established fact that the radium present in natural uranium minerals stands in constant ratio to the uranium. Rutherford and Boltwoodd have recently redetermined this ratio and found that one gram uranium is in radioactive equilibrium with 3.8 X 10-7 grams radium. If then the emanation from a small weighed quantity of a uranium mineral, whose percentage of uranium is known from a chemical analysis, be introduced into the electroscope, and the rate of fall of the leaf noted, the data are at hand from which a constant for the electroscope may be calculated that shall express the uranium (or radium) required to produced a fall of the leaf one division in unit time.
aAm. Jour. Sci., 4th ser., vol. 18, 1904, p. 381.
bJour. Phys. chem., vol. 9, 1905, p. 320; Trans. Am. Electrochem. Soc., vol. 8, 1905, p. 291.
cProc. Roy. Soc., vol. 77, 1903, p. 472.
dAm. Jour. Sci., 4th ser., vol. 22, 1906, p. 1.
The operation of standardizing was carried out as follows: The apparatus used for separating and collecting the emanation is shown in figure 2. A small quantity of a standard sample of powdered uraninite containing 68.9 per cent uranium was weighed out and put in the flask (F), of about 50 cubic centimeters capacity. Generally 15.7 milligrams of uraninite was used, as the emanation separated from this quantity of the mineral upon solution represents the total emanation associated with 10 milligrams uranium. A rubber stopper fitted with a small dropping funnel and a short delivery tube was then inserted in the flask and connections were made with the gas burette (B), which had previously been filled with freshly boiled distilled water, to which a few cubic centimeters of sodium hydroxide had been added. A few cubic centimeters of diluted nitric acid was then poured into the drop funnel; the leveling reservoir of the gas burette was lowered below the level of the acid in the funnel; and then by opening the pinchcock (c) and the stopcock of the funnel most of the acid was allowed to flow into the flask. The stopcock was then closed, the leveling reservoir replaced, and the flask gently heated until the uraninite had dissolved. When any gas collected in the stem of the funnel during this operation it was displaced by water introduced through the funnel. By withdrawing the flame for a moment sufficient water was allowed to flow into the flask to continue the boiling for ten minutes. The gas collected in the burette, after standing for ten minutes was then introduced into the electroscope, which had previously been partly exhausted, to be standardized. After three hours, the time in which the activity attains a maximum, the rate of leak was determined.

|
| FIGURE 2.—Apparatus for separating emanation from uraninite. |
The standardizing record of one of the electroscopes, which is designated in our journal as No. 1, is given below in Table 1. The quantity of uraninite used in each experiment was 15.7 milligrams. Determinations numbered 3 and 4 were conducted in Yellowstone Park in the field laboratory, and the others were made in the chemical laboratory of the University of Missouri. The samples of uraninite used for standardizing in the park were carefully weighed out and sealed in small glass tubes before we started for the field work. The first column in the table gives the number of the experiment; the second, the date on which it was conducted; the third, the number of scale divisions the leaf fell per minute at the time of maximum activity—three to three and one-half hours after the introduction of the gas. The normal air leak of the electroscope, 0.15 division per minute, has been deducted from the readings. The last column gives the constants computed for the electroscope. These values represent the quantity of uranium that would cause a fall of the leaf of one scale division per minute, if all of the emanation produced remained in the sample. In this particular sample of uraninite, 7.6 per cent of the emanation is continuously lost at room temperatures. The quantity of uraninite used in the experiments, however, 15.7 milligrams, contains the maximum quantity of emanation that would be found associated with 10 milligrams of curanium. The sample taken contains 10.8 milligrams of uranium. Hence the quantity of uranium responsible for a leak of one division per minute if all the emanation produced were retained is obtained by dividing 10 milligrams by the observed leak per minute.
TABLE 1.—Standardizing record of electroscope No. 1.
| Number. | Date. | Observed leak (divisions per minute). |
Uranium per division per minute (grams X 10-4). |
| 1 | June 28, 1905 | 44.1 | 2.27 |
| 2 | Aug. 15, 1903 | 45.0 | 2.22 |
| 3 | July 19, 1906 | 35.5 | 2.88 |
| 4 | Aug. 24, 1906 | 37.0 | 2.70 |
| 5 | Nov. 14, 1906 | 48.0 | 2.07 |
| 6 | Mar. 8, 1907 | 42.8 | 2.35 |
| 7 | May 22, 1907 | 45.7 | 2.18 |
The table shows that the constants obtained in the park are approximately 25 per cent higher than the average of the values obtained at Columbia, Mo. The lower density of the air at the higher altitude isan important cause of this difference. The value 2.8 X 10-4 was used for this electroscope in converting scale readings taken in the field to the uranium standard. The radium constant of the electroscope during the field tests was the product of 2.8 X 10-4 and 3.8 X 10-7, or 10.64 X 10-11.
In the field the constant for electroscope No. 2 was found to be 7.7 X 10-4 grams uranium and for electroscope No. 3 the value was 1.52 X 10-4 grams uranium for a leak of one scale division per minute.
ELECTRIC CAPACITY.
Inasmuch as some investigators, chiefly those on the continent of Europe, have expressed their quantitative results for the radioactivity of water and gas samples in electrostatic units, we have standardized two of our electroscopes in C. G. S. units. We are thereby enabled to express our values in absolute units and to compare the activities obtained with those given for some of the well-known thermal waters of foreign countries.
In expressing the radioactivity of waters, the standards proposed by Curie and Labordea and by Macheb have been generally adopted. The activity is the value obtained in electrostatic units for the saturation current produced by the radium emanation present per liter of water. For gases the saturation current produced by a liter of the gas is given as its radioactive value. Mache, in expressing the value of activities, deducted from the observed values the current due to the disintegration products of the radium emanation. The values given by Curie and Laborde represent approximately the total maximum activity, which includes the activity of the disintegration products of the emanation.
aCompt. Rend., vol. 138, p. 1150.
bSitzungsber. K. Akad. Wiss., Wien, Math-nat. Klasse. Abt. 2a, vol. 313, pp. 1329-1352.
To express the saturation current in electrostatic units two quantities must be determined—(1) the rate of fall of the leaf in volts per unit of time and (2) the electric capacity of the leaf system. In making our electroscopic readings we found the number of divisions the leaf fell per minute. To convert these readings into volts it is only necessary to calibrate the scale in the eyepiece of the reading microscope between the two points for which readings were generally made. This operation was conducted as follows: The glass plate on the back of the electroscope was replaced by one of cardboard, which was provided with an opening about 1.5 centimeters square, opposite the point where the leaf is attached to the carrying blade. The leaf system was then connected through the opening in the cardboard to one pole of a set of small storage batteries and the case of the electroscope to the other pole. The positions of the leaf corresponding to several voltages between 300 and 400 were then noted. In this way it was found that one small division of the scale of electroscope No. 1 represented 1.24 volts, and for electroscope No. 2 one division represented 2.80 volts. The electric capacity of the electroscopes was determined by means of a Harms standard condenser whose capacity was 42.5 centimeters. The procedure given by Harmsa was carefully followed. Connections with the leaf system were again made through the opening in the cardboard. The capacities of the instruments were small; that of electroscope No. 1 was found to be 2.81 centimeters and that of No. 2, 3.51 centimeters. The leaf system of electroscope No. 1 had the following dimensions: The aluminum leaf was 1.0 centimeter wide and 5.4 centimeters long, and it was fastened to a blade 0.9 centimeter wide and 6.2 centimeters long.
aPhys. Zeitschrift, vol. 5, pp. 49-50.
| <<< Previous | <<< Contents >>> | Next >>> |
bul/395/sec1.htm
Last Updated: 20-Nov-2007