
|
Geological Survey Bulletin 395
Radioactivity of the Thermal Waters of Yellowstone National Park |
METHODS OF PROCEDURE.
FIELD EXAMINATION OF GASES.
From many of the springs in the park gases escape with the issuing water. In some instances the evolution of gas from the hot water is so copious as to give the appearance of boiling. It is also not uncommon to see gases bubbling up in considerable quantity at certain points in the pools of hot water that dot the sinter plain in most of the different basins of thermal activity. Systematic tests were conducted with the escaping gases throughout the series of field experiments. The gas activity was generally the first determination made. It was conducted at the spring and consisted of two parts: (1) A qualitative test for the detection of thorium emanation as well as radium emanation; (2) a quantitative determination of the radium emanation present in the gas.
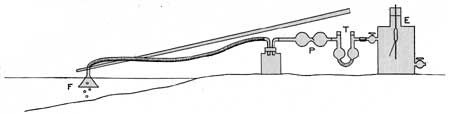
The principal parts of the apparatus used in the qualitative test are shown in figure 3: A glass collecting funnel (F) 12 centimeters in diameter, a rubber hand bellows (P), a drying tube (T) containing strong sulphuric acid, and one of the electroscopes (E) were connected in series by rubber tubing. By operating the hand bellows the water gas collecting in the funnel was gradually made to displace most of the original air in the electroscope. One to five minutes was generally required for this operation, the time depending upon the rate at which the gas collected in the funnel. When 1 to 2 liters of gas had been passed along the circuit, the pumping was stopped and the cocks of the electroscope closed. The leaf was then quickly charged and readings taken on its rate of fall by means of a stop watch and the reading microscope. By taking readings at suitable intervals for five to ten minutes the presence of thorium as well as radium emanation could be detected. Sometimes the leaf was charged before the transfer of gas through the electroscope was interrupted, so that readings could be taken at the instant the leaf appeared in the field of the microscope. By the latter procedure the relative activities of the thorium and radium emanation in the electroscope may be more readily obtained.
|
| FIGURE 3.—Apparatus for qualitative tests in the field. |
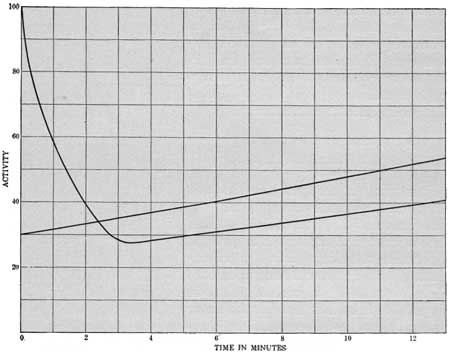
In figure 4 the points for curve A represent a series of readings obtained with a gas containing both thorium and radium emanation, and curve B represents the data for a gas containing only radium emanation. The activity is given in an arbitrary scale for the time in minutes. In the gas containing both emanations the activity drops very rapidly at first, falling to approximately half value in a minute—the characteristic rate of decay of thorium emanation. The activity remaining after five minutes mainly represents that of the radium emanation, showing its characteristic initial rise, as in curve B. After readings had been taken for ten minutes the gas in the electroscope was blown out with the hand bellows to avoid the accumulation of the disintegration products of the emanation. After half an hour the instrument was generally ready for another test.
|
| FIGURE 4.—Curves showing difference in the radioactivity of gases containing (A) thorium and radium emanation and (B) radium emanation. |
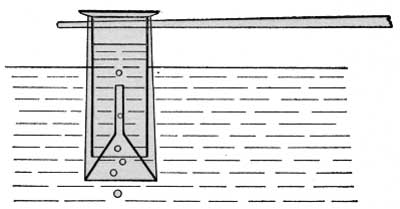
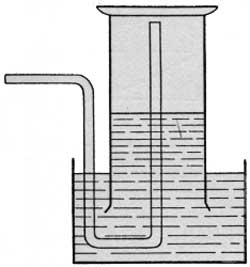
If the escaping gas was found to be active, a known volume was collected over water and this was introduced into an electroscope, which had previously been partially exhausted, for a quantitative determination of the radium emanation. The device generally employed for collecting the gas is shown in figure 5. A collecting funnel and a graduated cylinder attached to a pole were filled with water and then inverted over the escaping gas. When 50 to 100 cubic centimeters of gas had collected in the cylinder, it was brought within arm's reach, the funnel removed under water, the cylinder transferred to a small pneumatic trough, and the whole removed from the pool to cool. To hasten the cooling, the trough was oftentimes placed in a larger vessel of cold water. After ten minutes the temperature of the water was taken and the volume of the gas read. In the meantime a rubber tube supplied with a pinchcock was introduced up the cylinder, as shown in figure 6. The gas was then transferred to the partially exhausted electroscope with the drying train in circuit. The water gas remaining in the train and tubing was swept into the electroscope by admitting outside air until atmospheric pressure was restored. The rate of fall of the leaf was then taken at intervals during several hours following. The maximum activity, reached about three hours after the introduction of the gas, was made the basis of calculations in these measurements. Figure 7 represents the results obtained in one of these determinations. After four hours (point B on the curve) the gas was blown out. The rapid rate of decay of the induced activity, as well as the initial rise, are characteristic of radium emanation.
|
| FIGURE 5.—Apparatus for collecting gas in the field. |
|
| FIGURE 6.—Detail of apparatus for collecting gas in the field. |

|
| FIGURE 7.—Decay curve of radium emanation. |
FIELD TESTS OF WATERS.
In almost all the tests the determination of the activity of the water samples consisted in a quantitative measurement of the radium emanation in a fresh sample of water. The emanation dissolved in a known volume of water was separated by boiling in a form of apparatus which had been used in earlier investigations on the radioactivity of waters. The apparatus is figured and fully described in the Journal of Physical Chemistry, volume 9, pp. 324-326. Several parts of the apparatus were modified to adapt it to field conditions. Metal vessels were substituted for the glass ones formerly used as leveling reservoirs. The gas burette for measuring the volumes of the separated gases was rarely used. The heating was done with a small wood fire. The boilers used were provided with bails and also served as collecting vessels. A small quantity of caustic soda was generally added to the water sample before boiling to fix the carbon dioxide carried by the waters.
About ten minutes after boiling had ceased the separated gas was drawn into one of the standardized electroscopes, which had previously been partially exhausted by means of a hand suction pump. The activity was then ascertained in the usual way by noting the rate of fall of the charged leaf at intervals of fifteen to thirty minutes for several hours. The maximum leak, obtained three and one-half hours after the introduction of the gas, was made the basis for calculating the activity of the water sample.
The presence of radium emanation in a natural water does not necessarily indicate the presence of dissolved radium salts. Being a gas, the emanation found in a water sample may simply have diffused into it in the course of its underground flow. Such a water, however, has only temporary activity, for the emanation when separated from its parent radium decays rapidly, falling to half value in about four days, one-fourth value in eight days, and so on, until at the end of a month the activity has practically disappeared. If radium salts are present the water sample will still be radioactive after standing in a sealed vessel for a month and the residue after evaporation will also be radioactive. The presence of radium salts may be established by either of two methods—(1) by an examination of the residue obtained by evaporation, or (2) by storing the sample from which the emanation has been expelled by boiling, and at the end of a week or two again determining the emanation that has accumulated. As our facilities for storing waters were limited we evaporated the samples and reserved the residues for examination in the laboratory after our return from the park. The water was evaporated on a camp stove in iron bowls of about 2 liters capacity. The greater portion of the residue was removed with a spatula and the firmly adhering portion was removed by vigorous scouring with emery cloth, a fresh piece of cloth being used for each residue.
TESTING OF SOLIDS.
In determining the radium content of the spring deposits, water residues, and rock samples the method devised by Strutta in his investigations on the distribution of radium in the crust of the earth was followed in the main.
aProc. Roy. Soc., vol. 77, 1906; p. 472.
The calcareous deposits were dissolved in hydrochloric acid and the solution stored for several weeks in tightly corked flasks. The weights of material used in these determinations are given in the tabulated statement of results which appears further on. The slight quantities of insoluble residue were not removed; but were stored with the solutions. After the requisite period of storage the accumulated emanation was boiled off in an apparatus similar to the one used in the tests with water samples, except that the flask in which the solution was stored served as the boiler. The quantity of emanation was then determined, as it was for water and gas samples, by means of a standardized electroscope. With the siliceous deposits and the rock samples the determination of radium in small quantities involves more labor. The sample must first be ground to go through a 100-mesh sieve. Then it is fused with a mixture of alkali carbonates, the mass extracted with water, and the soluble portion stored in a tightly corked flask. The residue after it is dissolved in hydrochloric acid is stored in another flask. The activity of the two solutions is determined at the end of three or four weeks, as before described in connection with the water tests. From the data thus obtained the quantity of radium per gram of substance is readily calculated.

|
| PLATE II. NORRIS GEYSER BASIN. |
| <<< Previous | <<< Contents >>> | Next >>> |
bul/395/sec2.htm
Last Updated: 20-Nov-2007