|
CUMBERLAND ISLAND
An Ecological Survey of the Coastal Region of Georgia NPS Scientific Monograph No. 3 |

|
CHAPTER 3:
The Islands
Georgia meets the Atlantic Ocean along the beaches of the offshore islands, popularly known as the sea islands or the Golden Isles. Scientists call them barrier islands because they form a barrier between the sea and the land. Barrier islands occur along the Atlantic and Gulf coasts from New Jersey to Texas. A chain of southern sea islands having similar natural and cultural histories occurs from North Island, S.C., to Anastasia Island, Fla. The Georgia islands are several miles offshore and are separated from the mainland by extensive marshland, tidewater streams, and sounds.
Other islands, in addition to the barrier islands, are scattered throughout the estuarine systems. These islands are of various origins. Some of the hammock (forested) islands are remnants of old barrier islands formed in the past during periods of higher sea level (Hoyt et al. 1964). Others may have been separated from larger islands by erosion (Teal and Teal 1964). Some of the small islands are thought by many to have been formed from ballast dumped by ships (Emery et al. 1968). Most of the marsh islands are alluvial deposits dissected and shaped by estuarine drainage systems.
This chapter deals primarily with the barrier islands: their formation, floral and faunal composition, and functional ecology. The barrier island system of Georgia consists of eight island complexes including 13 barrier islands (Fig. 1). Table 4 shows the length of the beach and the area, excluding salt marsh, of each of the barrier islands.
Elevations on the barrier islands typically range from sea level to about 25 ft, although individual dunes may be higher. (Some on Cumberland are over 50 ft high according to maps of the U.S. Geological Survey.) Topography of the islands is typically characterized by broad, nearly level areas interspersed with low, gently sloping ridges. Islands and portions of islands that are of more recent origin may be characterized by steep, parallel dune ridges (especially Wassaw, Blackbeard, and Little Cumberland). The entire coastal region of Georgia is mapped by the U.S. Geological Survey at three different scales: 1:24,000 (7.5 quadrangles), 1:62,500 (15' quadrangles), and 1:250,000 (1° lat. by 2° long.).
Upland soils are mostly porous sands derived from recently deposited marine sediments that are resistant to weathering (Regosols). These soils have a distinct A horizon (surface layer) with significant accumulations of organic matter that accounts for most of the exchange capacity (Byrd et al. 1961). They are subject to moderate to severe leaching, and many are excessively drained. Principal soil series include Blanton, Galestown, Klej, Lakeland, and Palm Beach (Byrd et al. 1961). Lower, poorly drained sites are characterized by intrazonal soils of the following series: Leon, Ona, Plummer, Rutlege, and St. Johns (Byrd et al. 1961 and unpublished soil survey information). Most of these soil series are characteristically very acid, but locally on the islands they may be neutral to slightly alkaline due to the presence of oyster shells in the profile.
TABLE 4. Areas of Georgia islands and lengths of their beaches.a
| Island | Approximate acreageb |
Approximate miles of beach |
| Tybee | 1,500 | 3.4 |
| Little Tybee | 1,600 | 3.0 |
| Wassaw | 2,500 | 6.0 |
| Ossabaw | 11,800 | 9.5 |
| St. Catherines | 7,200 | 11.0 |
| Blackbeard | 3,900 | 7.5 |
| Sapelo | 10,900 | 5.6 |
| Little St. Simons | 2,300 | 6.5 |
| Sea | 1,200 | 4.7 |
| St. Simons | 12,300 | 3.8 |
| Jekyll | 4,400 | 8.0 |
| Little Cumberland | 1,600 | 2.4 |
| Cumberland | 15,100 | 16.9 |
aFrom U.S. Geological Survey maps. bIncludes forested area, pastures, beaches and dunes, and freshwater marsh and ponds. Does not include salt marsh. | ||
Of the 13 barrier islands, only Blackbeard has not been in private ownership in this century. Acquired by the Navy Department in 1800 for its live oak forests, it is now a national wildlife refuge. All or parts of several other islands have passed into public ownership in recent years. Jekyll Island was purchased by the State of Georgia in 1947 for intensive public recreation. A portion of Sapelo Island was purchased by the Georgia Game and Fish Commission in 1969, and Wassaw was acquired by the Bureau of Sport Fisheries and Wildlife in 1969. A major portion of Cumberland Island has been acquired for a national seashore pending enactment of authorizing legislation.
Of the islands in private ownership, Tybee, St. Simons, and Sea islands are in multiple ownership and have been intensively developed as residential and recreational areas. Part of Sapelo owned by the Sapelo Island Research Foundation is the site of the University of Georgia Marine Institute, where much of the research on which this report is based was conducted. The remaining privately owned islands are used for vacation retreats, wildlife refuges, commercial livestock production, and research and education in cooperation with educational institutions in the state.
Despite their wild appearance, the Georgia islands are not undisturbed. Most of them have been under intensive agriculture at one time. Timber has been harvested from all except Wassaw, although a few small virgin stands remain on some of the islands. Vegetation on several of the islands has been modified by large numbers of hoofed animals. Many native animals have been extirpated and exotic species have been introduced.
Barrier island formation1
Although barrier islands border the Atlantic coast and many other coasts that are tectonically stable, the way in which these islands form is not known. They formed many thousands of years ago; direct observation of their origin and development is not possible, but several theories have developed. A widely accepted theory proposed by a Frenchman over a century ago was that waves coming from the open ocean into shallow water become steeper, and when they break they lose energy and dump some of the sand they have been carrying, forming a bar which grows until it reaches high water level (Beaumont 1845). Eventually there is a time, this theory proposes, when dunes develop on top of this bar to form an island (Fig. 4).
1This section is contributed by the late John H. Hoyt, and is modified from a previous publication (Hoyt 1968c).
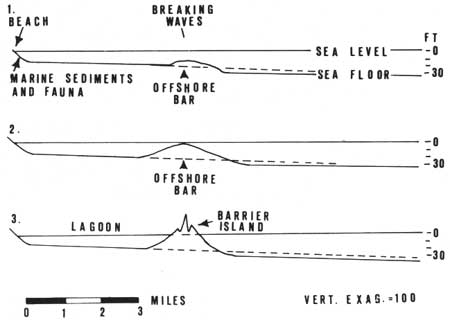
|
| Fig. 4. Idealized cross-sections showing barrier island formation from an offshore bar. 1. Waves agitate sea floor and deposit sediment to form bar in area of energy loss. 2. Sediment accumulates to near sea level. 3. Bar is converted to island with lagoon on landward side. (From Hoyt 1968c.) |
Recent geological studies indicated that there were several things wrong with this theory. One was that during the early stages of bar formation there must have been an open marine beach in the area behind the bar. However, the shells, fauna, and sediment that should be in this location, if this theory were correct, were never found during drilling on Sapelo Island (Hoyt et al. 1964) and elsewhere (Bernard et al. 1959; Fish 1959; Shepard 1960; Shepard and Moore 1960; Rusnak 1960; Straaten 1965). It was finally concluded that the barrier islands probably did not form in this way.
The formation of the glaciers that covered North America and Europe during the Pleistocene ice ages affected the ocean by withdrawing a large quantity of water. At the maximum extent of ice formation, sea level was probably as much as 330 ft lower than it is now and the seashore of Georgia was 70-80 miles eastward of its present location. The ice started to melt about 18,000 years ago, returning water to the ocean, and the level of the sea rose to its present position.
It was concluded that dune formation, which occurs nearly everywhere along the sand beaches, followed by flooding of the area by sea water, is a more reasonable explanation of the formation of barrier islands (Hoyt 1967, 1968a,b). Initially a series of dune ridges forms along the shoreline. If the dunes are stable and large enough, they are not destroyed during a submergence of about 20 ft. The area landward of the dunes is flooded and becomes a lagoon, which eventually fills with sediment and forms a salt marsh (Fig. 5).
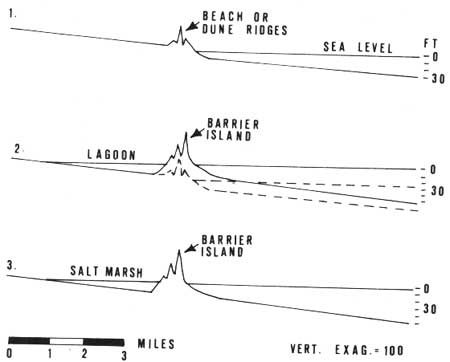
|
| Fig. 5. Formation of barrier islands by submergence. 1. Beach or dune ridges form adjacent to shoreline. 2. Rising sea level results in inundation of areas landward of ridge to form barrier island and lagoon. (From Hoyt 1968c.) |
The coastal islands are of two ages. Sea Island, for instance, is quite young geologically. It was probably formed within the last 4000-5000 years. St. Simons Island, just to the west, is older, having formed during the Silver Bluff submergence of the Pleistocene. Sea Island and the other Holocene islands have very poorly developed soil profiles because there has not been time for stratification to occur. St. Simons Island, however, has a mature soil profile with an extensive accumulation of humic matter at a depth of 3-8 feet. The age of the islands can be shown on a graph (Fig. 6) which indicates the relation of time to the altitude of the sea (Hoyt et al. 1968). Five samples of shells from sediments of the Silver Bluff submergence, analyzed by the carbon-14 method, were estimated to be 36,000-25,000 years old. These ages give a rough idea of the time the main parts of Sapelo and St. Simons islands formed. The seaward parts of these islands have formed within the last 4000-5000 years (Holocene). Figure 7 indicates which islands and parts of islands are Silver Bluff (Pleistocene) in age and which are modern (Holocene).
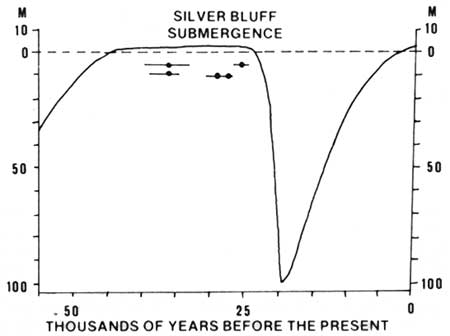
|
| Fig. 6. Diagram showing altitude of sea level during past 55,000 years. Control points are from carbon-14 dates of shells from Sapelo Island. Length of horizontal line represents age uncertainty. Sea level altitudes from 18,000 years to present compiled from several published sources. (From Hoyt 1968c.) |
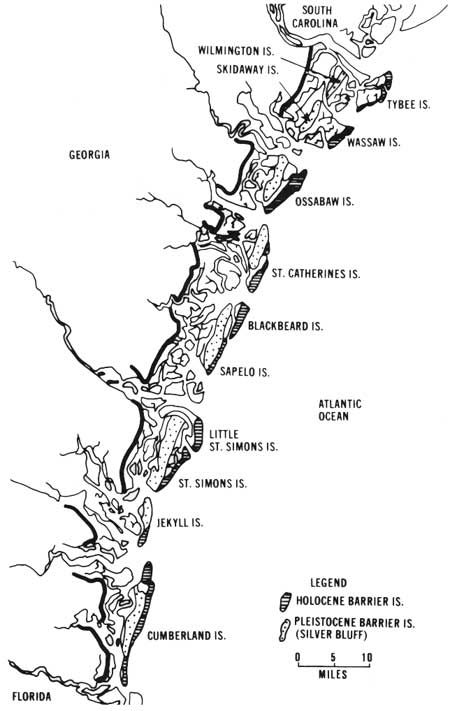
|
| Fig. 7. Geologic ages of the barrier islands of Georgia. (From Hoyt 1968c.) |
The geologic development of the lower Georgia coast is the result of barrier island formation during stages of the Pleistocene when sea level was higher than it is now. The oldest series of islands formed when sea level was as much as 100 feet higher than it is now (Wicomico shoreline). At that time a great barrier island, now called Trail Ridge, formed in front of the area now known as the Okefenokee Swamp (Fig. 8), which was then a very large salt marsh. The Wicomico barriers can be traced across coastal Georgia. They are discontinuous now but were formerly more extensive.
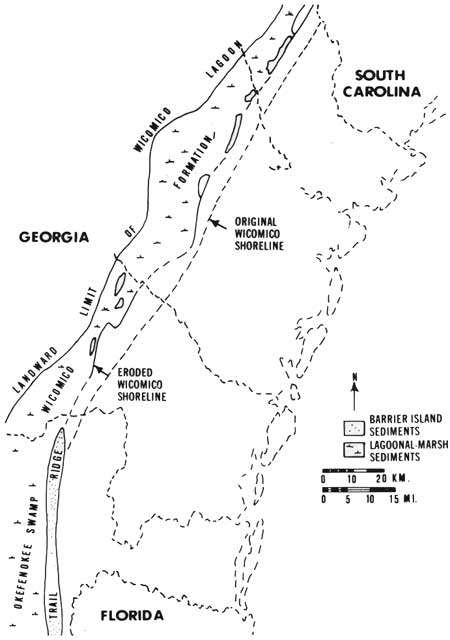
|
| Fig. 8. Locations of former barrier island and salt marshes along eroded Wicomico shoreline (sea level altitude 95-100 ft). Inferred location of preerosion shoreline also indicated. (From Hoyt 1968c.) |
Subsequent to the development of the Wicomico barrier, there was another period of ice formation and melting, and another sequence of barrier islands developed at a slightly lower altitude (about 75 ft above present sea level) known as the Penholoway shoreline (Fig. 9). Five subsequent fluctuations of sea level resulted in five additional systems of barrier islands now above sea level and traceable across coastal Georgia: Talbot, 40-45 ft; Pamlico, 25 ft; Princess Anne, 15 ft; Silver Bluff, 5 ft; and Holocene (Hoyt and Hails 1967) (Figs. 10,11).
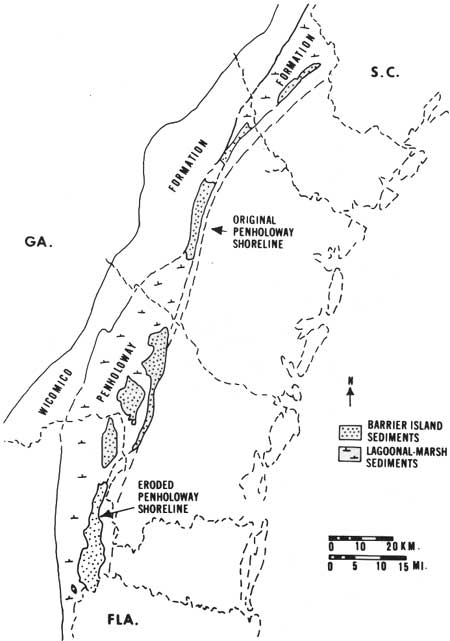
|
| Fig. 9. Locations of former barrier islands and salt marshes along eroded Penholoway shoreline (sea level altitude, 70-75 ft). Inferred location of preerosion shoreline also indicated. (From Hoyt 1968c.) |
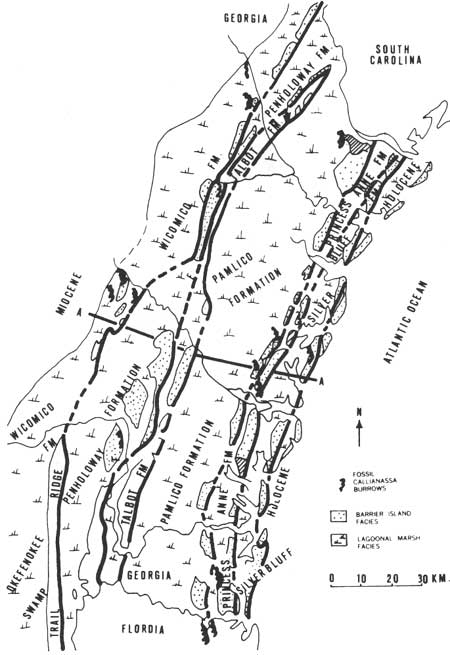
|
| Fig. 10. Location of barrier island and lagoonal-salt marsh sediments for six sequences of Pleistocene coastal deposits and for the Holocene deposits. (From Hoyt 1968c.) |

|
| Fig. 11. Cross-section of Pleistocene and Holocene sediments of coastal Georgia. Location of cross-section shown on Fig. 10. (From Hoyt 1968c.) |
Beaches and dunes
Physical features
The seaward side of Georgia's barrier islands is made up of fine quartz sands (Greaves 1966) which form the beaches and dune ridges. The beach is the gently sloping shore which is washed by waves and tides. It extends from the normal high tide line to 30 ft below the low tide line. The beach and dune area is commonly characterized by the following zones (Fig. 12): (1) Shoreface—the narrow zone seaward from the low tide shore line permanently covered by water over which the beach sands and gravels move with wave action. (2) Foreshore—the lower shore zone between ordinary low and high water levels. (3) Backshore—the upper shore zone beyond the reach of ordinary waves and tides extending to the base of the dunes. (4) Dunes—ridges of windblown (eolian) sand.

|
| Fig. 12. Typical profile of beach and dunes showing zonation. |
The beaches and dunes are constantly being resculptured by the action of waves, currents, and wind, which interact to keep the beaches and dunes in a dynamic state. Baldwin and Lofton described six beach types used as nesting sites by loggerhead sea turtles on Cape Island, S.C. (Caldwell 1959). These types, described below and in Fig. 13, are also geologically descriptive of those occurring in coastal Georgia. Many, if not all, of the types may occur on each barrier island. The dynamic state of the beaches and dunes often produces a gradation from one type to another.
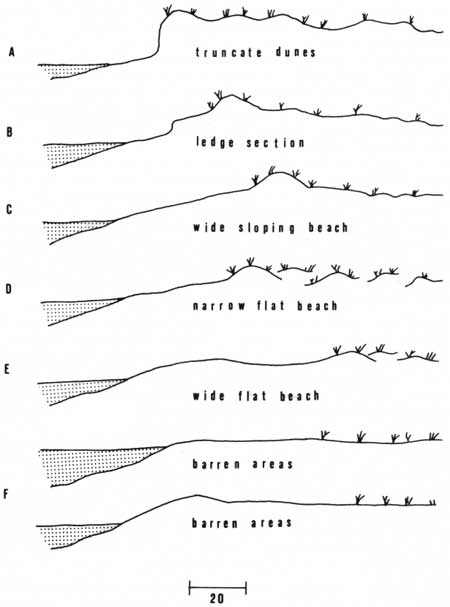
|
| Fig. 13. Diagrammatic cross-sections of generalized beach types occurring on the barrier islands. See text for explanation. (Adapted from Caldwell 1959.) |
A. Truncate dunes: Sharply eroded dunes backing a beach 5-10 ft wide on an average high tide. Unusually high tides erode the base of these dunes.
B. Ledge section: A stretch of beach having a 0.5-3 ft ledge breaking the middle of its natural slope. This type is formed by the action of wind and tide (undertow) and is variable.
C. Wide sloping beach: Twenty-five to 40 ft wide from average high tide line to base of dunes. The outer dune forms a continuous ridge paralleling the ocean front and is broken in only a few places.
D. Narrow flat beach: Ten to 20 ft wide and backed by small separated dunes.
E. Wide flat beach: Similar to the narrow flat beach, 30-50 ft wide and backed by small isolated dunes.
F. Barren areas: Stretching 100-400 ft back from the crest of the beach, with only traces of vegetation or low dunes to break their flatness.
EFFECT OF WAVES AND CURRENTS ON BEACH MORPHOLOGY AND STRUCTURE
Waves are formed when water particles are set into orbital motion by wind action. The height (amplitude) and energy of waves depend on the velocity and duration of wind and the extent of open water across which the wind can blow (fetch). The maximum fetch for the Georgia coast is 4000 miles to the east-northeast (Greaves 1966).
The Georgia beaches occur in a region of moderate wave energy—the lowest recorded along the southeastern Atlantic coast (Tanner 1960). The average height of breaking waves on the Georgia coast is 9-12 inches (Helle 1958). Because of a gently sloping terrace, water deepens gradually along the Georgia coast and much wave energy is dissipated before reaching the beaches.
As the waves enter shallow water, the drag of the bottom distorts the orbital motion of water molecules, shortens the wave length and the wave becomes steeper and forms a crest. The cresting wave breaks when the water depth is one to two times the amplitude of the wave. The more gradual the slope of the beach and continental slope, the farther offshore the waves will break.
The turbulence in the breaker zone and the swash of waves on the beach constantly rearrange sand particles and reshape the shoreline. Materials stirred up by waves are deposited as offshore bars just inland from the zone of greatest breakers. Sand grains are repeatedly carried onto the beach by the swash and carried back out with the backwash.
Lateral bars and troughs (ridges and runnels) commonly develop on the lower foreshore. Troughs on Sapelo Island are 20-40 ft wide and retain water at low tide (Greaves 1966). They apparently are formed by breaking waves. Troughs are separated by bars 0.5-3 ft high and 50-100 ft wide which are formed, at least in part, of materials from the troughs (Greaves 1966). The seaward slope of bars is usually less than 20, whereas the landward slope is considerably steeper (up to 300) (Hoyt 1962; Hoyt and Hails 1967).
The foreshore is commonly marked by water ripples of various types. Backwash ripples on the upper foreshore have rounded crests and narrow troughs. Oscillation ripples have sharp, symmetrical crests with broader troughs and occur in troughs (runnels) on the foreshore (Hoyt et al. 1966). Current ripples are produced by current flowing steadily out of troughs at low tide (Hoyt et al. 1966). They have a long, gentle slope on the upper side and a shorter, steeper slope on the lee side. Rhomboid ripples occur along crests of bars and are formed by water flowing landward over the bar (Hoyt and Henry 1963).
Several features are produced by the entrapment of air in the sands of the upper foreshore. Bubbled sand results in a very soft beach surface (Hoyt and Henry 1964), and sand domes about 4 inches in diameter are produced when trapped air forces up a thin layer of surface sand (Hoyt et al. 1966).
On beaches in other areas there are major seasonal changes in beach profiles. During the summer, when wave energies are lowest, many sand grains are not moved out with the backwash and there is a net movement of sand landward. This results in the gradual buildup of sand on the backshore. A horizontal bed of sand (a berm) extends from the foot of the dunes to a pronounced beach ridge at the high-tide mark. The berm area serves as a source of sand for replenishment and growth of the dunes. In the fall and winter wave energy is greater, the berm erodes, and there is a net movement of sand from the beach to the breaker zone where it is deposited as an offshore bar.
But on most Georgia beaches the berm is poorly formed because seasonal changes in wave energy are relatively slight. The backshore typically slopes gradually, and there is no pronounced beach ridge. There is no consistent difference in slope between winter and summer beaches (Pilkey and Richter 1964), although there is a constant exchange of sand between the dunes, the beach, and the offshore bar.
There is also a longshore movement of sand caused by littoral currents. A pattern of north-to-south sediment transport is well documented (e.g., Hoyt et al. 1964; Greaves 1966; Frey and Howard 1969; and others). Islands are eroding (Fig. 14) on the north and aggrading on the south. For example, the erosional process is evidenced in Fig. 15 by the sharply truncated dune ridges on the north end of Blackbeard Island. The sediment from the erosion is transported southward and deposited at the south end of Sapelo Island where beaches are prograding and dunes are increasing in size. Thus, the islands are shifting southward. Hoyt and Henry (1967) estimate a southward advance of Sapelo island of about 0.75 mile during the present (Holocene) high stand of the sea. The rate of movement varies and may be much greater on some islands.

|
| Fig. 14. Aerial photograph of acute beach erosion on the northern end of Wassaw Island, May 1970. |
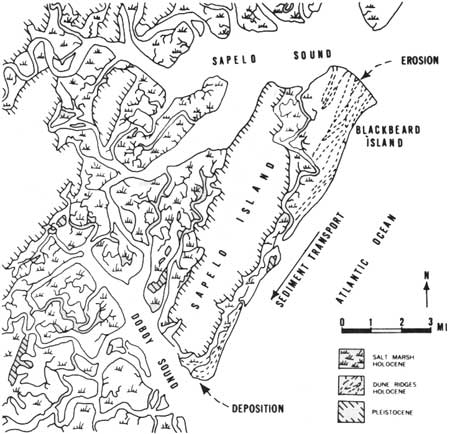
|
| Fig. 15. Patterns of sediment deposition, erosion, and transport, Sapelo Island and vicinity. (From Hoyt 1968c.) |
DUNE FORMATION
Dunes form as a result of windblown sand piling up behind minor obstacles. Once started, the dune itself becomes an obstacle to windblown sand, and the lodgment of more sand causes the dune to grow. Dunes and dune ridges along the Georgia coast normally grow to 10 or 12 ft in height (occasionally much higher) and acquire a distinct morphology characterized by gentle windward and steeper leeward slopes. Surface ripples parallel the dune ridge at right angles to the wind.
Vegetation plays an important part in the formation and stabilization of dunes. Salt-resistant beach plants trap windblown sand, forming little mounds of sand or dunelets which grow as the plants respond with increased growth and trap more sand. These foredune plants must have the ability to withstand salt spray, roots that will endure exposure, and stems that will withstand burial by shifting sands. They must be perennials able to keep above the sand, spread laterally, and withstand drought (Cowles 1899).
Sea oats (Uniola paniculata) is the most important dune-former on the Georgia coast (Fig. 16). Sometimes sea beach panic grass (Panicum amarum) also may form substantial dunes. Other species produce only low, temporary dunes because they lack a sufficient lateral root system, have excessive water requirements, or lack the ability to keep above the sand (Kurz 1942). Wagner (1964) conducted detailed studies on the ecology of sea oats and their role in the formation of dunes. The following discussion is based upon his work.

|
| Fig. 16. Sea oats (Uniola paniculata), an important plant in dune stabilization. (Photograph by Merry M. Tyler.) |
Spikelets fall from the plant late in the fall and early in the winter and are rapidly disseminated by the wind. Spikelets falling on sites of sand accretion are rapidly buried. (Most of those falling on stable sites are eaten by birds and mammals.) Seedlings are produced in the spring and become established during the first growing season. During the second season, extensive tillering occurs. For several years thereafter plants put on vigorous growth, followed by flowering. Colonization is by windblown seed dispersal followed by tillering to enlarge the colony. Sea oats trap windblown sand and respond to the sand deposition with vigorous growth of rhizomes and vertical shoots. This stimulates further growth and the process continues until dunes form in a series of parallel ridges. Sea oats are replaced by other vegetation when the dunes become partially stabilized.
Dunes cease vertical accretion when they have reached such a height that wind no longer can transport sand to the top. New dunes then form to the windward (seaward) side. Thus, the largest dunes are farthest inland, sheltering the forest behind.
Even partially vegetated dunes are subject to blowouts and shifting. Erosion of dunes is accelerated by grazing, human activity, or other disturbance. When the sand on the windward slope is not anchored, it is continually carried over the top by the wind and deposited on the lee side, resulting in migrating or "marching" dunes. Dunes may protect the forest during its formation only to bury it later (Oosting 1954) (Fig. 17). Oosting and Billings (1942) reported that grazing transformed several of the banks in North Carolina into almost barren seas of shifting sand. Similar damage from grazing has been reported from South Carolina and Texas (Johnson 1900: Cottam 1970) and is evident on several islands along the Georgia coast (Fig. 18). Storms may breach the dunes, especially where erosion has occurred, creating blowouts and unstable terraces and damaging trees and shrubs (Oosting 1954; Wagner 1964).

|
| Fig. 17. Large dune encroaching upon interior forest on Cumberland Island. Height of the dune is approximately 45 ft, June 1971. |

|
| Fig. 18. Top. Natural dune system on Blackbeard Island, June 1971. Dunes are well-stablized by sea oats and other vegetation. Bottom. Severely overgrazed beachfront on Cumberland Island, June 1971. Overgrazing has resulted in the loss of dune vegetation with the subsequent loss of the dune system resulting in a wide, flat beachfront. Extremely high tides (storm induced) may inundate the entire beachfront and erode the older, well-established dune systems protecting the island interior. |
COMPOSITION OF BEACH AND DUNE SAND
Constant washing and grinding wears down sand grains and sorts them as to size, resulting in a lamination of beach particles.
A regional sorting of materials might be expected as a result of the southward movement of sediments. Studies indicate, however, that the composition of beach sands is primarily related to source materials and regional wave energy (Giles and Pilkey 1965; Giles 1966). Sands of Georgia beaches and dunes are of finer median grain size than those farther north and south, apparently because higher wave energies transport coarser sands (Giles and Pilkey 1965; Giles 1966).
Samples of sands from Georgia beaches contained virtually no calcium carbonate, whereas this was an important component of beach sands to the north and south (Giles and Pilkey 1965; Giles 1966). This too is apparently a result of the inability of waves of low energy to transport, fragment, and abrade shells (Giles and Pilkey 1965; Giles 1966). Dunes along Georgia beaches contain relatively few shell fragments.
Heavy minerals in sands of Georgia beaches have been analyzed by Neiheisel (1962, 1965, 1966), Giles and Pilkey (1965), and Giles (1966). The most prevalent heavy minerals on beaches at Jekyll Island are ilmenite, zircon, and epidote (Neiheisel 1962). Other components of the heavy mineral fraction of beach sands are as follows: tourmaline, sillimanite, kyanite, staurolite, hornblende, garnet, rutile, leucoxene, and monazite (Neiheisel 1962). Neiheisel (1962) showed that heavy minerals in sands on Jekyll Island are evidenced as (1) thin, narrow, black surface seams concentrated in the upper littoral zone; (2) thin horizontal beds of black sand extending shoreward beneath dunes; (3) cross-bedded lamination in dunes; and (4) disseminated particles in beach and dune sediments. The distribution of heavy minerals as a result of selective sorting by waves is determined largely by mineral shape and density. Heavier minerals occur in greater concentration lower on the beach (Neiheisel 1962). On Jekyll Island the heavy mineral fraction comprised 50-90% of enriched concentrate on the upper beach, 5-30% of the laminations and thin bands in the foredunes, and only 4-5% disseminated in average dunes (Neiheisel 1962).
SOURCES OF BEACH AND DUNE SAND
The principal sources of heavy minerals and sands on the Georgia coast are (I) the Altamaha and Savannah watersheds which originate in the Piedmont and mountain areas of the state; (2) the smaller Coastal Plain watersheds that are of more recent origin; and (3) suspended material from the continental shelf.
Heavy minerals of the beaches and dunes more closely resemble assemblages from the Piedmont rivers than they do assemblages from Coastal Plain rivers (Giles 1966). This suggests that Coastal Plain rivers are not important contributors to present beach sediments (Giles 1966).
There is also an apparent relationship between the composition of beach sands and the mineralogy of the adjacent continental shelf, and continental shelf material is another important sediment source (Giles and Pilkey 1965; Giles 1966; Levy 1968).
The salt spray community of plants
Few species of vascular plants can survive the extremely harsh physical environment of the beaches and dunes. In order to inhabit this area, plants must possess characteristics enabling them to withstand the combined effects of salt spray, constant wind, full light intensity, high evaporation, and high temperatures. They must be capable of becoming established in and keeping above the shifting sands. Distance from the surf and location relative to dunes or protective vegetation on the seaward side will determine the exposure of a site to these limiting factors. Thus there is a gradient or, more commonly as a result of the modifying effect of the dunes, a zonation of vegetation from mean high tide toward the interior of the island.
Most ecologists attribute this zonation to the effects of salt spray or to the combined effects of salt spray and wind (Wells and Shunk 1937; Wells 1942; Doutt 1941; Boyce 1954). Studies by Boyce (1951,1954) revealed that salt spray forms as a result of droplets of sea water being forcefully ejected from the crests of waves and the bursting of bubbles during effervescence of foam. These droplets are concentrated by evaporation and transported inland by winds as a salt spray to be deposited on sands and vegetation. Droplets deposited on the surface of the sands remain there until rain leaches the salts out of the porous sand. Consequently, there is no significant salinity in dune soils and little chloride is absorbed through the roots of plants. There is apparently no correlation between soil salinities and plant distribution on the dunes (Oosting and Billings 1942; Boyce 1954). Boyce concludes that the limiting effect of salt spray results from direct deposition on the foliage and that the plants best adapted to the salt spray are those with characteristics that minimize deposition and absorption of salts.
Zones of beach vegetation have been classified in various ways (Wells 1942; Boyce 1954; Oosting and Billings 1942; Oosting 1954). Although this progression of vegetation types is commonly referred to as successional, the concept of succession is probably not applicable to the beach-dune environment because of the overwhelming effects of harsh physical factors. Apparent successional relationships between zones are not primarily a result of reaction of plants on habitat (Oosting 1954).
No studies of beach and dune vegetation have been reported from Georgia, and the discussion of vegetation that follows is based upon the authors' observations in Georgia, published reports from North Carolina (Wells and Shunk 1937; Oosting and Billings 1942; Wells 1942; Doutt 1941; Oosting 1945; Boyce 1951,1954; Oosting 1954; Wagner 1964) and Florida (Kurz 1942), and unpublished lists of plants from several Georgia islands.
Plants occurring on the beach include sea rocket (Cakile spp.); beach hogwort (Croton punctatus); beach sandspur (Cenchrus tribuloides); salt meadow cordgrass (Spartina patens); salt wort (Salsola kali); sea-purslane (Sesuvium spp.); beach-spurge (Euphorbia polygonifolia) and seashore-elder (Iva imbricata). Principal plants of the foredunes are sea oats, sea beach panic grass, railroad vine (Ipomoea stolonifera), beach pennywort (Hydrocotyle bonariensis), Spanish-bayonet (Yucca spp.), and some of the plants of the beach (e.g., seashore elder, beach spurge, and sea rocket). Annuals such as camphorweed (Heterotheca subaxillaris) may temporarily colonize dunes until killed out by salt spray.
The foreslope and the crest of the foredunes are subject to the greatest intensity of salt spray. Little salt is deposited on the lee slope of the foredunes or in the interdune area. In these areas principal species include, in addition to some of the species previously mentioned, little bluestem (Andropogon scoparius), prickly pear (Opuntia spp.), seaside goldenrod (Solidago sempervirens), beach primrose (Oenothera humifusa), juniper (Juniperus virginiana), yaupon (Ilex vomitoria), wax myrtle (Myrica cerifera), and live oak (Quercus virginiana).
Bluestem occupies the drier sites. Low, flat areas behind breaks in the fore dunes that are periodically inundated by unusually high tides may be occupied by stands of salt meadow cordgrass (Oosting and Billings 1942; Oosting 1945).
Salt spray, after passing over the interdune area, next contacts the windward slope of the rear dunes and is deposited on the vegetation that occurs there. Consequently, sea oats and other salt-tolerant plants of the windward slope of the foredunes are also dominant there. Behind the crest of the rear dunes, sites are more protected and vegetation is more diverse. Shrubs and trees may dominate this area. Trees and shrubs most commonly occurring in this zone are live oak, red bay (Persea borbonia), wax myrtle, juniper, yaupon, cabbage palm (Sabal palmetto), saw palmetto (Serenoa repens), and groundselbush (Baccharis halimifolia).
Shrubs and trees are commonly pruned by the wind and salt spray, producing a sloping, sheared effect (Fig. 19). Studies by Boyce (1954) have shed light on the mechanism by which the salt spray produces this effect. Salt enters the leaves through abrasions caused by the lashing of wind action. High chloride ion concentration produces necrosis and death of exposed leaves and branches. Chloride ions are transported to the leaf apices and twigs and accumulate there. They are not translocated to the leeward side of the tree in injurious quantities, so only the windward sides of the plants are killed, producing an asymmetrical form. Pruning stimulates vigorous sprouting which results in the rapid formation of a dense canopy that reduces the efficiency of deposition on the plant and on the individual stems. Most dune plants have a uniformly closed crown which is less efficient in accumulating salt deposition. Projecting limbs subject to greater salt accumulation are killed. Mechanical action of winds that prune and bend leaves and branches and high transpiration rates' augment the effects of salt spray.

|
| Fig. 19. Live oaks pruned by wind and salt spray on St. Catherines Island. (Photograph by H. N. Neuhauser). |
Wells and Shunk (1937, 1938) listed characteristics of the live oak that are advantageous in a salt spray environment, and Wells (1939) designated the live oak forest as a "salt spray climax," but this concept has been strongly challenged (Oosting 1954; Laessle and Monk 1961).
Animals and food webs
THE TIDAL BEACH
The monograph of Pearse at al. (1942) described the ecology of tidal beaches in North Carolina and listed the inhabitants of beach zones. Studies of the beach fauna in Georgia are limited to a survey of isopods (Frankenberg and Menzies 1966), a study of amphipods and associated fauna (Croker 1967), and studies of species that leave tracks and trails in the geologic record (Frey and Howard 1969). Table 5 is a compilation of the species listed in these three works. It is by no means complete or comprehensive, but it may serve as a preliminary listing of the major infauna.
Sand beaches are harsh environments requiring highly specialized adaptations. They are areas between land and sea that are alternately flooded and drained. While flooded, the substratum may be drastically rearranged, and inhabitants are preyed upon by predators from the sea. When drained, at low tide, the beach is exposed to the desiccating effects of sun and wind, and inhabitants are preyed upon by predators from the land.
Permanent inhabitants burrow into the sands at low tide, thus avoiding desiccation and exposure to predation. Many other species use the beach as a feeding ground, either occupying it when it is covered with water (e.g., fish, crabs) or foraging there as the tide recedes (e.g., shorebirds).
The distribution of burrowing forms is greatly influenced by the size of sand grains which determines pore volume and thus moisture and oxygen content. There is a zonal segregation where tides ebb and flow. The coarsest particles are found where wave energy is greatest. Finer sediments occur above and below the tidal zone. Consequently, there is a zonation of burrowing fauna (Pearse et al. 1942). Because of low wave energies, the median size of sand grains on Georgia beaches is smaller than on beaches to the north and south (Giles and Pilkey 1965).
Burrowing forms usually lack pigment, have reduced sense organs, and have highly specialized adaptations that allow burrowing and feeding (Pearse et al. 1942). Burrow structures (lebensspuren) are abundant, especially in the lower foreshore. The more distinctive ones are produced by ghost shrimp (Callianassa sp.), mole crabs (Lepidopa sp.), sandworms (Class Polychaeta), razor clams (Tagelus sp.), acorn worms (Phylum Hemichordata), and sea cucumbers (Synapta sp.) (Frey and Howard 1969). The deep burrows of ghost shrimp are especially evident and have been useful in ascertaining Pleistocene sea levels on the Georgia coast (Weimer and Hoyt 1964).
More active inhabitants include various gastropods, sand dollars (Mellita sp.), and brittle stars (Class Ophiuroidea) (Frey and Howard 1969).
In the spring and early summer, horseshoe crabs (Limulus polyphemus) appear on the beaches in large numbers to breed and lay their eggs. The female scoops out a shallow depression in the moist sand slightly below or at the high-tide line in which she deposits 200-300 eggs, which are immediately fertilized by the male. The female then quickly covers the nest and the pair returns to the surf (Lochhead 1950). Late in the summer tiny crabs may be seen on the beach sand at low tide. The breeding and reproductive stage of the life cycle is the only time horseshoe crabs come ashore. Both young and adults normally feed in the bottom habitat of shallow waters. Some of the largest individuals of the species have been reported from the Georgia coast (Shuster 1955).
TABLE 5. Some in fauna of barrier beaches in Georgia.a
| Organism | Tidal zoneb |
| Phylum Cnidaria (Coelenterata) | SF |
| Sagartia lacteus | |
| Phylum Nemertinea | |
| Cerabratulus lacteus | SF-LF |
| Micrura sp. | |
| Phylum Sipunculoidea | |
| (unidentified) | mid-FS |
| Phylum Mollusca | |
| Class Pelycepoda | |
| Dinocardium robustum | SF |
| Donax variabilis | LF-UF |
| Mactra fragilis | LF-UF |
| Tagelus divisus | SF |
| Tagelus pleveius | SF |
| Tellina agilis | SF |
| Class Gastropoda | |
| Busycon carica | SF-LF |
| Busycon perversum | SF-LF |
| Oliva sayana | SF-LF |
| Polinices duplicatus | SF-LF |
| Sinum perspectivum | SF-LF |
| Terebra dislocata | SF-LF |
| Phylum Annelida | |
| Class Ollgochaeta | |
| (unidentified) | mid-FS |
| Class Polychaeta | |
| Diopatra cuprea | SF-LF |
| Heteromastus sp. | SF |
| Laonereis culveri | UF |
| Nephthys picta | SF-LF |
| Onuphus microcephala | SF-LF |
| Owenia fusiformis | SF |
| Paraonis sp. | SF |
| Pectinaria gouldii | SF |
| Scololepsis sp. | FS |
| Scoloplos sp. | UF |
| Spiochaetopterus oculatus | SF |
| Streblospio benidicti | SF |
| Phylum Arthropoda | |
| Class Crustacea | |
| Order Cumacea | |
| Bodotriid | SF |
| Order Isopoda | |
| Ancinus depressus | |
| Chiridotea caeca | |
| Cyathura polita | |
| Exosphaeroma diminutum | |
| Sphaeroma quadridentatum | |
| Order Amphipoda | |
| Acanthohaustorius sp. | LF |
| Corophium sp. | SF |
| Haustorius sp. | UF |
| Lepidactylus dytiscus | mid-FS |
| Monoculodes sp. | FS |
| Neohaustorius schmitzi | FS |
| Parahaustorius longimerus | LF |
| Protohaustonus deichmannae | SF |
| Pseudohaustorius caroliniensis | SF |
| Talorchestia sp. | UF |
| Order Decapoda | |
| Callianassa major | SF-UF |
| Callianassa atlantica | SF-LF |
| Emerita talpoida | SF |
| Lepidopa websteri | LF |
| Ocypode quadrata | UF-BS |
| Ogyrides alphaerostris | SF |
| Pagurus longicarpus | SF |
| Pinnixa chaetopterana | SF |
| Phylum Echinodermata | |
| brittle stars | SF |
| Mellita quinquiesperforata | SF-LF |
| Synapta inhaerens | SF |
| Phylum Hemichordata | |
| Saccoglossus kowalevskii | SF |
| Balanoglossus biminiensis | SF |
aCompiled from Frankenberg and Menzies (1966), Croker (1967), and Frey and Howard (1969). bBS = backshore; FS = foreshore; LF = lower foreshore; SF = shoreface; UF = upper foreshore. | |
There is considerable nutrient exchange between the beach and the sea. Much organic matter of marine origin, especially macroscopic algae, is washed up on the beach. Brown algae (Phaeophyta) of the genus Sargassum may virtually cover the beach after high winds (Caldwell 1968). Additional organic matter is contributed by cordgrass (Spartina) stems and bird droppings. This organic matter is acted upon by bacteria, which are abundant in the beach sands (Pearse et al. 1942). The products of bacterial decomposition are dissolved and returned to the sea. The bacteria themselves form one of the bases of the food chain; being fed upon by copepods, nematodes, flatworms, protozoa, and amphipods (Pearse et al. 1942). Another important base of the food chain involving beach animals is one-celled algae.
Beach invertebrates are predators, plankton-feeders, or detritus-feeders. Many plankton- and detritus-feeders have bristle-like structures (setae) on the legs (Callianassa), the antennae (Emerita), or mouth parts (Lepidopa) for collecting small particles of organic matter (Pearse et al. 1942). Burrowing clams (e.g., Donax) siphon water through the mantle cavity and catch organisms on threads of slime (Pearse et al. 1942). Other animals (e.g., Balanoglossus) ingest large quantities of sand, digesting the organic matter, and passing the remainder through the alimentary canal (Pearse et al. 1942).
Carnivores are typically more mobile and include marine animals that follow the rising tide (silversides2, killifish, flounders, lizard fish, crabs, creeping star fish) and terrestrial predators that feed on the exposed beach at low tide (Pearse et al. 1942).
2Scientific names of vertebrates can be found in Appendices 1-5.
Among those birds which feed on mollusks, crustaceans, and other invertebrates at low tide are the American oyster-catcher, semipalmated plover, pipping plover, Wilson's plover, black-bellied plover, ruddy turnstone, willet, American knot, least sandpiper, semipalmated sandpiper, western sandpiper, and sanderling. Several species feed below and in the intertidal zone on small fish. These include common terns, least terns, royal terns, and black skimmers. Herring gulls, ring-billed gulls, black vultures, and fish crows are common scavengers of the beach and dune areas. Formerly, the bald eagle was a commonly observed scavenger along the beach.
UPPER BEACH AND DUNES
The area of the beach above the high-tide mark is inhabited or frequented primarily by organisms with terrestrial affinities, but some marine types inhabit or use the area.
Various arthropod predators and scavengers of terrestrial origin forage along the drift line. These include tiger beetles (Cicindelidae), rove beetles (Staphylinidae), earwigs (Dermaptera), springtails (Collembola), and spiders (Pearse et al. 1942).
Most conspicuous among the invertebrates of this zone is the ghost crab or sand crab (Ocypode quadrata). Although of marine origin, the adult ghost crab lives in burrows along the upper beach and well back into the dunes. Primarily nocturnal, the ghost crab forages as a scavenger and predator.
The upper beach also serves as a nesting area for certain species. Loggerhead turtles, with ancient evolutionary ties to the terrestrial environment, instinctively return to the beach to lay eggs. This important species will be discussed in a separate section to follow.
Several species of birds nest on the upper beaches. These include royal tern, least tern, American oyster-catcher, Wilson's plover, and willet. Also black skimmers and gull-billed terns have been reported to nest on Little Egg Island, a "sandbar island" in the mouth of the Altamaha River.
Except for the loggerhead turtle and the ghost crab, animals using the dunes are terrestrial. Food habits data of animals from dune areas are lacking, but it is evident that many species forage in the dunes.
One of the few permanent mammal residents of the dune area is the eastern mole, one of the most common mammals on some islands. Its extensive runways are very common in the dunes, and occasionally they extend to below the high-tide line. Moles are generally insectivorous, although a certain amount of plant material occasionally may be consumed (Golley 1962).
Sea oats, in addition to their important role in dune formation and stabilization, also form an integral part of the food web involving important animals characteristic of the habitat. Tippins and Beshear (1968) reported scale insects (Duplasionaspis) infesting sea oats, and Circulaspis and Odonaspis seriously affecting other dune grasses (Spartina and Panicum). Wagner (1964) reported 15 species of beetles and three species of sucking bugs associated with sea oats panicles during flowering. Collops nigriceps and Isomira sp. were especially common. He examined the stomach contents of seven individuals of each of these species and found that all contained pollen grains of sea oats.
Wagner (1964) also examined the stomachs of 11 house mice. Eight contained seeds of sea oats; 10 contained bluestem seed; and two contained arthropod fragments. There were no Peromyscus on Bogue Bank, N.C., where Wagner conducted his studies, but he cited Nelson (1918) as reporting that the old field mouse feeds extensively on sea oats in Florida.
Both the old field mouse and the cotton mouse occur on some, but not all of the Georgia islands. On Cumberland Island cotton mice commonly forage in the dunes. Their tracks may be seen during the early morning hours leading from one clump of vegetation to the next. On the mainland the cotton mouse is usually found in relatively moist habitats, and the old field mouse is more common in drier habitat (Golley 1962). Few data exist on the food habits of either species on the islands.
The marsh rabbit is a common animal frequenting the dune area. Droppings are frequently observed in the sheltered area behind the dunes. Exact food habits of marsh rabbits are not known, but cut stems on Sapelo and Blackbeard islands indicated that they were feeding on sea oats.
Songbirds, especially song sparrows and other fringillids, and red-winged blackbirds are the major consumers of the seed of sea oats. Sea beach panic grass, which produces a panicle of large seed, is no doubt a very important source of food for seed-eating birds and mammals, although data are lacking. Beach hogwort and beach primrose probably also contribute to the diet of these birds.
Trees and shrubs that grow in the lee of the rear dunes produce large quantities of fruit and seed that are heavily utilized by a variety of animals including such larger, important species as wild turkeys, raccoons, deer, and hogs. Notable among these plants are live oak, wax myrtle, saw palmetto, yaupon, and dahoon (Ilex cassine).
Hogs visit the dunes to forage on beach pennywort. Cattle graze sea oats and other grasses and forbs on the dunes and move onto the open beaches at night to avoid mosquitoes. The activities of cattle and hogs are very detrimental to dune stability, as previously pointed out.
THE LOGGERHEAD SEA TURTLE
The Atlantic loggerhead sea turtle is a conspicuous element of the beach fauna in summer when mature females emerge from the sea to lay their eggs on the beaches.
The nesting range of the loggerhead is restricted primarily to the temperate zone (Caldwell et al. 1955) on barrier island and mainland beaches from Texas to Cape Lookout, N.C. (Caldwell et al. 1959). The loggerhead concentrates its nesting activities in specific areas and on specific beaches along a coastline. These nesting areas are commonly termed "rookeries." Two of the six largest rookeries of the Atlantic states are located on the Georgia coast: one on Blackbeard and the other on Little Cumberland and Cumberland islands.
Caldwell (1962) reported that loggerheads nested on St. Simons Island prior to the severe erosion of the seaward side of the island during the late 1950s, after which nesting declined considerably. It is not known if the turtles moved to the beaches of nearby Jekyll and Little Cumberland islands.
Prior to 1959, the beaches of Jekyll Island were used extensively by nesting loggerheads. However, following the development of Jekyll Island into a public recreation area in 1959, most loggerheads apparently moved to the beaches of nearby Little Cumberland and Cumberland islands (Caldwell 1962).
Little Cumberland and Cumberland islands support a large and important rookery today. The female population of Little Cumberland Island is currently estimated at 600-800, approximately one-third of which nests each year (J. I. Richardson, pers comm.). The total number of females using the Cumberland-Little Cumberland rookery is probably just under 1000 individuals.
Records indicate that Sapelo Island has not been used extensively for nesting in several years (Caldwell et al. 1959). Aerial surveys in 1970 indicated little use of the Sapelo beach.
Blackbeard Island has been the site of considerable nesting of loggerheads for many years. During 1969, 221 nests were recorded (W. L. Towns, pers. comm.).
The beaches on Little St. Simons, St. Catherines, Ossabaw, Wassaw, Wolf, and Tybee islands are used to a lesser degree. Our aerial survey of these beaches in May 1970 indicated that, except for the rookery beaches, the beaches of Little St. Simons and Ossabaw islands were used by the turtles more than the others.
Baldwin and Lofton reported that the "wide sloping beach" (Fig. 13C) was the most preferred nesting beach on Cape Island, S.C. (Caldwell 1959). They reported that turtles could easily crawl from the surf to the base of the dunes to nest. Caldwell (1959) noted that the nesting turtles along the Georgia coast preferred a "beach backed by high dunes or vegetation." The currently used beaches of Little Cumberland Island, Cumberland Island (portions), and Blackbeard Island are mainly of the "wide sloping beach" type.
The truncate dunes beach type (Fig. 13A) occurs on the rookery beaches of Georgia as well as on most other beaches. Normal high tides frequently inundate many nests located on this beach type, and therefore exert selection pressures decreasing the frequency of turtle nesting on the truncate dunes beach type.
The truncate dunes beach often grades into the "ledge section" beach (Fig. 13B). This type of beach often presents a physical deterrent to female turtles attempting to nest. The female loggerheads often turn back to the sea if they encounter this ledge (Caldwell 1959).
A wide sloping beach backed by high dunes practically insures the female loggerhead a safe return to the sea after nesting, as the unbroken dunes act as a deterrent to any inland travel. Many female turtles often become quite disoriented in their attempts to return to the sea after nesting on other types of beaches. Those females that nest on a beach backed by broken dunes (Fig. 13D, E) often crawl behind the dunes after nesting and frequently become hopelessly lost and die in the heat of the following day (Caldwell 1959). A similar fate often awaits those females that attempt to nest beyond the crest on the barren beaches (Fig. 13F). These females are often beyond sight of the sea after nesting and may wander for hundreds of feet in search of the surf. Many are not successful. A nesting site located at the base of the unbroken dunes on a wide sloping beach also assists the hatchling loggerheads in the necessary orientation to the surf (Daniel and Smith 1947; Caldwell 1959).
Natural processes acting on beach and dune formations produce constant change of many nesting beaches. Those beaches used by nesting turtles over a long period of time are usually characterized by stable dunes. An example of changes in dune types influencing nesting is given by Baldwin and Lofton (Caldwell 1959). They reported that a section of the Cape Island beach backed by little-used truncate dunes in 1939 was changed into the much preferred wide sloping beach in 1940. Since then a large number of turtles have been using them.
Nest destruction is high throughout the loggerhead's range. Natural predators destroy large numbers of nests and, in many areas, depredation by man is a serious limiting factor on reproduction. Man also has introduced hogs into some nesting areas, and they often are serious nest predators. Finally, natural and artificially induced beach erosion destroys many nests.
Sand crabs and raccoons are the principal natural nest predators in Georgia and in many other areas. Both have an uncanny ability to locate sea turtle nests. Usually, sand crabs are the first to arrive at a nest. They normally dig small holes down into the nest cavity and bring several eggs to the surface. The eggs are then eaten at the nest or transported to the nearby burrow of the crab. Baldwin and Lofton (Caldwell 1959) found that sand crabs entered 40.8% of the nests on Cape Island, S.C., but did not destroy all of those nests.
Raccoons are the most common natural vertebrate predators of turtle nests (Carr 1967) (Fig. 20). They are common even on the most isolated of the barrier beaches. On Cape Island, S.C., Baldwin and Lofton (Caldwell 1959) found that raccoons patrolled the beaches during the nesting season singly or in family groups and that relatively few individuals were responsible for nest depredation. These individuals covered the same area throughout the summer.

|
| Fig. 20. Top. Loggerhead sea turtle nest destroyed by raccoons on Blackbeard Island, 23 June 1971. Bottom. Wire shield placed over sea turtle nest to retard raccoon depredation, Blackbeard Island. |
On many of Georgia's barrier islands, both European wild hogs and domestic (now feral) hogs have been introduced. On some beaches these animals are serious nest predators of sea turtles (Carr 1967). McAtee (1934) wrote that "the work of natural enemies is insignificant compared to the depredation of hogs where they are present." J. I. Richardson (pers. comm.) states that, because of nest depredation by feral hogs and raccoons, natural reproduction of sea turtles on Little Cumberland Island has been almost nil. However, the conservation-minded owners of Little Cumberland maintain an artificial hatchery which increases hatching success to approximately 60-80% (6000-10,000 young turtles per year).
Cumberland Island has a large feral hog population. Cumberland also has a large nesting population of turtles and approximately 16 miles of nesting beach, yet it probably produces fewer turtles per unit of beach than any other island.
Ossabaw and St. Catherines islands also have numerous feral hogs that no doubt seriously reduce natural nest production. Forty-five hogs were counted on the beaches of Ossabaw during a morning flight in May 1970. Several groups of hogs were concentrated at the apex of turtle crawls made the previous night. Their behavior and signs of excavation indicated that they were feeding in a turtle nest.
Erosion by storms and strong shoreward winds during high tides frequently washes out turtle nests. However, these natural factors probably do not seriously affect the long-term population dynamics of sea turtles. Baldwin and Lofton found that most washing occurred on the low beach below the truncate dunes, a site least preferred for nesting on Cape Island, S.C. (Caldwell 1959).
Human destruction of nests is very common on those nesting beaches with easy access and, to a lesser degree, on those beaches with limited access. Dietary motivation ("turtle egging"), malicious recreation ("turtle egg fights," LeBuff 1969), and general curiosity are responsible for the destruction of many nests. Fortunately, the Georgia rookeries receive excellent protection from human depredation due to the conscientious efforts of some of the private landowners and the efforts of personnel of the Georgia Game and Fish Commission and of the U.S. Fish and Wildlife Service on Blackbeard Island and other nesting beaches. However, nests located on many of the barrier island beaches cannot be adequately protected from human depredation because of the limited manpower available for patrolling the extensive area involved.
The development of several of Georgia's beaches for recreational purposes has seriously reduced the amount of nesting beaches along the coast. While some beaches may not be altered physically, the construction of highways and buildings nearby deters female loggerheads from nesting (Caldwell 1962; Carr 1967), and also emerging hatchlings often become disoriented by the artificial illumination of highways and buildings and move toward these sources of light instead of the sea (Caldwell 1962; Caldwell and Caldwell 1962). Carr (1967) reported that thousands of young loggerheads are crushed on some of the coastal highways adjacent to nesting beaches, and many others succumb the following day to desiccation and predation.
Savannah Beach has been totally destroyed as a nesting beach by development; a sea wall now replaces the dunes. Both natural and man-induced erosion has destroyed the nesting beach on St. Simons Island. Very few turtles nest on St. Simons today. The development of Jekyll Island, site of a former rookery, apparently influenced the movement of the rookery to nearby Little Cumberland Island. The development of Jekyll included both the destruction of the beaches and dunes and the construction of beachside motels and other buildings. The destruction of the beaches created a physical impediment to nesting, and the illumination of the motels deterred many females from nesting on beaches that were still physically acceptable.
The island interior
Forest associations and successional relationships
Despite much study of the forests of the southeastern Coastal Plain, only the pine forests are well understood. Little is known about the maritime forests. Except for the compilation of species lists (which have not been published), there have been no studies of the forest vegetation of the Georgia islands. Bourdeau and Oosting (1959) described the structure of the maritime forest in North Carolina, but the geology of the North Carolina islands differs somewhat from the Georgia islands, and some of the subtropical plants that are very important on the Georgia islands are not present in North Carolina. Coker (1905) briefly described the forests on the Isle of Palms near Charleston, S.C. Laessle and Monk (1961) reported on some live oak forests of northeastern Florida. Otherwise, there have been no relevant studies of areas ecologically comparable to the Georgia islands. Consequently, much of the discussion of the forest vegetation of the Georgia islands is based on our observations and interpretations. The major forest types on the Georgia islands are live oak, pine, mixed hardwood, cypress-gum, and bay. Other less discrete associations may be recognized.
The maritime live oak forest is characterized by a distinct dominance of live oak (Fig. 21). Sandy soils of old dune ridges on the seaward side of the islands support small, slow-growing live oaks of poor form. Cabbage palms frequently intrude into the canopy, and an understory of saw palmetto, vacciniums, and other species of low woody vegetation may be present. Where exposed to winds from the sea, the closed canopy usually forms a continuous sloping surface which affords protection of the interior of the forest from the winds and salt spray.

|
| Fig. 21. Live oak forest, Cumberland Island, June 1971. Note absence of Spanish moss. Many old stands have more dense canopy and woody understory. |
Size of the oaks increases toward the interior of the island, and the oaks develop large trunks and huge, spreading crowns. The diversity of species is also much greater in the interior of the island. Some important woody components of the community are listed in Table 6.
Conspicuous features of the maritime live oak forest are an abundance of sclerophyllous (leathery-leaved) broad-leaf evergreens, lianas, and epiphytes, and relatively few herbaceous plants.
Spanish moss (Tillandsia usneoides) drapes the larger trees, especially the live oaks, obtaining nutrients from dust particles and from particles in the water that drains onto it from the limbs above. Ecologically and aesthetically, Spanish moss is probably the second most important plant in the live oak forest, the most important being the live oak itself. Draping the spreading crown of the live oak, Spanish moss significantly reduces the amount of light that penetrates the canopy and is to a large extent responsible for the dark, humid atmosphere beneath the canopy and, consequently, the composition of the understory vegetation. The moss provides nesting habitat for at least three species of songbirds: parula and yellow-throated warblers and painted buntings (Teal and Teal 1964). It provides nest-building material for many other birds. At least two species of bats (Lasiurus seminolus and L. intermedius) commonly use Spanish moss for roosting sites (Barbour and Davis 1969). The moss provides habitat for many kinds of insects important in the food chain. Rainwater (1941) reported 164 species of arthropods associated with Spanish moss.
TABLE 6. Some important woody plants of live oak forests on the barrier islands of Georgia.
| Common name | Scientific name |
| Trees and shrubs | |
| Loblolly pine | Pinus taeda |
| Juniper | Juniperus virginiana |
| Switchcane | Arundinaria tecta |
| Saw palmetto | Serenoa repens |
| Cabbage palm | Sabal palmetto |
| Wax myrtle | Myrica cerifera |
| Live oak | Quercus virginiana |
| Water oak | Quercus nigra |
| Laurel oak | Quercus laurifolia |
| Sweet bay | Magnolia virginiana |
| Southern magnolia | Magnolia grandiflora |
| Red bay | Persea borbonia |
| Hercules'-club | Zanthoxylum clava-herculis |
| American holly | Ilex opaca |
| Dahoon | Ilex cassine |
| Yaupon | Ilex vomitoria |
| Red buckeye | Aesculus pavia |
| Devil's walking stick | Aralia spinosa |
| Sparkleberry | Vaccinium arboreum |
| Devilwood | Osmanthus americana |
| Beauty-berry | Callicarpa americana |
Woody vines | |
| Smilax | Smilax spp. |
| Poison ivy | Rhus radicans |
| Virginia creeper | Parthenocissus quinquefolia |
| Grape | Vitis spp. |
| Pepper-vine | Ampelopsis arborea |
| Rattanvine | Berchemia scandens |
| Yellow jessamine | Gelsemium sempervirens |
| Coral honeysuckle | Lonicera sempervirens |
Beginning about 1967, serious mortality of Spanish moss was noted in Florida (Edward Allen, Report of Smithsonian Center for Short-lived Phenomena, 27 December 1967) and along the Georgia coast. In the summer of 1970 most of the moss along the coast south of Liberty County was apparently dead (Fig. 22). The mortality did not extend far inland in Georgia but extended southward to Jacksonville, Fla., and across Florida to Tampa. The cause of this mortality is not definitely known, and we have been unable to establish whether similar mortality has occurred in the past. A common pathogenic fungus (Fusarium solani) has been implicated (Roberts et al. 1971). Pollution has been suspected as a contributory factor, but this has not been demonstrated. Although new growth is evident and the Spanish moss appears to be recovering, this situation needs to be seriously studied.

|
| Fig. 22. Top. Healthy Spanish moss growing on live oak on St. Simons Island, 1960. Bottom. Same area, July 1970. Note absence of Spanish moss. (Photographs by Merry M. Tyler.) |
Other epiphytes—lichens, mosses, and ferns—grow in abundance in the organic matter that accumulates in the deep fissures of the bark on the nearly horizontal branches of the oaks. Most conspicuous among these is the resurrection fern (Polypodium polypodioides). The number of species of epiphytic bryophytes increases with increasing age of the forest (Mackaness 1942).
Little is known about successional processes of the island forests. Wells (1939) contended that the live oak becomes dominant only near the coast where salt spray reduces competition from other species. He noted that the maritime live oak forest is a long-lived, relatively stable community even on mature soils capable of supporting "ordinary broad-leaved climax species of the region." Consequently, he designated the maritime live oak forest a "salt-spray climax." Oosting (1954) questioned this concept on the grounds that the sequence of vegetative types that develops on a site is determined not by the reaction of the community on the site, but by physical factors such as wind and salt spray and the topographic position of the site. Laessle and Monk (1961) concluded that inland climax species can exist under the influence of salt spray in Florida, and that the live oak forest is not a salt-spray climax in Florida.
Because of its tolerance to salt spray, xeric conditions, and infertile soils, live oak is commonly the first forest community to become established on coastal sand dunes. Thus established with this competitive advantage, the live oak forest apparently persists indefinitely and appears to be a near-climax community. Even as the island grows seaward by the formation of new dunes and the site becomes relatively protected from the influence of salt spray, the live oak forest withstands all competition and is extremely resistant to change.
Infrequent fires of low intensity may contribute to the maintenance of live oak dominance (Laessle and Monk 1961). Live oaks are very susceptible to fire (Fowells 1965), and their occurrence on the mainland is restricted to areas protected from frequent, intense fires (Heyward 1939; Laessle and Monk 1961). But on the islands the initial establishment of live oaks occurs without being preceded by a "fire-type" (pine) community. Islands in general are relatively more isolated from wild fires (Harper 1911), and the likelihood of a stand burning is related to the size of the island. Furthermore, the sand dunes, where the live oak forests develop, are often somewhat isolated from fires that do occur on the island and usually do not have enough ground cover to support fires. As the trees mature under such conditions, they become less susceptible to destruction by fire, and the live oak forest itself acquires characteristics that protect it from fire. The high humidity, relatively sparse ground cover and fire resistant litter beneath the mature live oak stand do not allow fires of such intensity as to bring about a decline in live oak dominance.
Thus, the maritime live oak forest is a long-lived, near-climax community that becomes established as a result of an interaction of physical factors that reduce competition from other species and protect the community from fire. Once established, this forest type is quite stable and resistant to change because of the long lifespan of the tree, its ability to sprout prolifically and its adaptation to site characteristics. Also, occasional fires may deter invasion by climax species (Laessle and Monk 1961).
The pine forest typically occurs on well-drained sites protected from sea winds where original live oak forests have been cleared for agricultural or other purposes and secondary succession has proceeded along lines more typical of mainland forests (from herbaceous pioneers to pine forests). The pine type with its more open canopy allows development of herbaceous ground cover and the formation of a mat of needles which together provide fuel for frequent fires. The effects of periodic burning are favorable to the maintenance of a predominantly pine stand (e.g., Garren 1943; Chapman 1950).
Even where fire is excluded on the islands, it is questionable if secondary succession, in the absence of salt spray and other uncommon circumstances relating to soils and moisture, leads to a climax dominated by live oak. More likely, succession is toward an association of mixed hardwoods with a number of codominants (the southern mixed hardwood forest of Quarterman and Keever 1962) (Fig. 23).

|
| Fig. 23. Mature hickory stand on Cumberland Island. Small stands of hickory occur on several of the barrier islands. Their origin and former distribution on the islands are not known, but they probably are the climax of secondary succession on certain sites (whereas the live oak forest may be the product of primary succession). Other associations of mixed hardwoods also occur on the islands. |
The pine forests on the islands are dominated by loblolly pine or, less commonly, slash pine (Pinus elliottii) or longleaf pine (P. palustris). Pond pine (P. serotina) occurs on wet, acid sites but usually does not attain dominance. Pine forests that are frequently burned are generally open, with a ground cover of grasses and herbaceous plants and a rather scattered understory of saw palmetto, wax myrtle, beauty-berry, various species of Ilex and Vaccinium and other small trees and shrubs tolerant of fire. The open aspect of the pine woods is further developed on some islands by large numbers of grazing animals (Fig. 24). In the absence of fire and grazing, a dense, tangled understory of woody vegetation develops that generally is less productive habitat for most species of wildlife. Some deliberate (prescribed) burning is done on the islands to accomplish certain management objectives such as reducing undesirable competition in stands of merchantable timber, improving wildlife habitat and livestock range, reducing fire hazard (fuel), and improving access, visibility, and aesthetic appeal.

|
| Fig. 24. Open pine forest and pastures grazed by cattle on St. Catherines Island. |
Very wet sites support communities of wax myrtle, buttonbush (Cephalanthus occidentalis), willow (Salix sp.), gum (Nyssa sp.), red maple (Acer rubrum), ash (Fraxinus sp.), and occasionally cypress (Taxodium ascendens). Succession is toward a bay community dominated by such species as loblolly bay (Gordonia lasianthus), sweet bay, and red bay.
Ponds and sloughs
Low areas between dune ridges on the islands commonly form sloughs containing fresh or slightly brackish water. These ponds and sloughs play a major role in maintaining some of the more interesting wildlife of the islands, notably alligators and wading birds.
The sloughs vary considerably in size and depth. Some dry up completely in summer; others contain water throughout the year. The water is usually acid and stained so that light penetrates only 2 or 3 ft below the surface. Consequently, if the sloughs are deep, there may be relatively little growth of submersed aquatics and an abundance of emergent plants, rooted plants with floating leaves, and unrooted floating plants. (see Table 7 for common species.)
Sloughs are not static communities (Fig. 25). Succession and eutrophication lead to cat-tail marsh and ultimately to forested swamp. Fire, the intrusion of salt water on hurricane tides, and fluctuating water levels may alter vegetative composition and temporarily set the community back to an earlier successional stage. During prolonged dry periods sloughs may be invaded by cypress and gum.

|
| Fig. 25. Top. Slough of recent origin behind dunes on Cumberland Island, June 1971. Bottom. Slough in interior of St. Catherines Island, May 1971. (Photographs by H. N. Neuhauser.) |
In the fall, sloughs are important resting and feeding areas for migrating and wintering waterfowl which feed upon various parts of water-shield, pondweed, bushy pondweed, bulrushes, the introduced banana water-lily, and other aquatic plants. Acorns and other kinds of mast from adjacent trees also provide much food for waterfowl. (See Table 12 for significance of specific aquatic plants as waterfowl foods.)
In the spring, large numbers of herons, egrets, and other wading birds form nesting colonies in the sloughs. (See Appendix 4 for locations and descriptions of rookeries on the Georgia coast.) Large numbers of nestlings fall into the water providing food for alligators and cottonmouths, and dense populations of these reptiles may build up in sloughs having large colonies of nesting birds (Wharton 1969; Teal and Teal 1964).
TABLE 7. Some common plants of sloughs and ponds on Georgia islands.
| Common name | Scientific name |
| Submerged | |
| Bushy-pondweed | Najas spp. |
| Fanwort | Cabomba caroliniana |
| Coontail | Ceratophyllum demersum |
| Parrot's-feather | Myriophyllum brasiliense |
| Floating-leaved, rooted | |
| Pondweed | Potamogeton spp. |
| Water-shield | Brasenia schreberi |
| Banana water-lily | Nymphaea mexicana |
| White water-lily | Nymphaea odorata |
| Yellow cow-lily | Nuphar luteum |
| Floating, not rooted | |
| Mosquito fern | Azolla caroliniana |
| Duckweed | Lemna spp. |
| Duckweed | Spirodela spp. |
| Water-meal | Wolffia spp. |
| Wolffiella | Wolffiella floridana |
| Bladderwort | Utricularia spp. |
| Emergent | |
| Cat-tail | Typha spp. |
| Bulrush | Scirpus spp. |
| Rush | Juncus spp. |
| Sawgrass | Cladium spp. |
| Pickerelweed | Pontederia cordata |
| Arrow arum | Peltandra virginica |
| Alligator-weed | Alternanthera philoxeroides |
| Arrowhead | Sagittaria latifolia |
Alligators and most of the amphibians and freshwater turtles are dependent upon the sloughs for survival. The smaller sloughs that are periodically dry are especially important breeding habitat for amphibians because of the absence of predatory fish. The importance of the island sloughs in the preservation of the endangered American alligator is discussed in another section.
Food webs and nutrient cycling
Forest vegetation utilizes solar energy to convert carbon dioxide and water to simple sugars. These and certain minerals from the soil are metabolized in plants to produce various fats, proteins, and carbohydrates which enter the food web when the green plants are consumed by other organisms. These organisms are fed upon by secondary consumers, and the energy and nutrients are thus passed through the ecosystem. Fallen leaves and branches are acted on by fungi and microorganisms. Nutrients in solution enter the root systems of plants and are thus continually recycled. Energy, however, is not recycled because some energy is converted to heat and radiated into the atmosphere at each transfer. Thus the number of transfers is limited.
Much of the solar energy reaching the mature live oak community is intercepted in the canopy by live oaks and other large trees, lianas, and Spanish moss and other epiphytes. Of that which does penetrate the canopy, most is intercepted by an understory of small trees, shrubs, and woody vines; little reaches the forest floor. Consequently, in a mature live oak forest, photosynthesis of trees and shrubs accounts for most energy conversion. This energy and minerals from the soil enter the food web by way of decomposers (especially fungi), invertebrate consumers of wood, foliage and other plant parts, a few grazers and browsers, and many fruit-eating birds and mammals.
The live oak community includes an abundance of woody species that produce fruit and mast, which is important in the diet of the omnivorous birds and mammals inhabiting this habitat. Live oak acorns are probably the most important single food for many species of wildlife including deer, raccoons, feral hogs, wild turkeys, and other birds. Other important fruit- and seed-producing trees and shrubs providing food for these animals include laurel oak, saw palmetto, smilax, American holly, yaupon, dahoon, wax myrtle, vacciniums, red bay, sweet bay, southern magnolia, beautyberry, rattanvine, Virginia creeper, and muscadines and other wild grapes.
In the pine type relatively little light is intercepted by the canopy and, especially where fire is a frequent factor, there is an abundance of ground story vegetation (especially grasses). In this habitat primary consumers are more likely to be grazers (especially insects), browsers, and small seed-eaters.
Larger, more mobile animals commonly use both the live oak and the pine forests and especially the ecotone where the two types meet.
Insectivorous birds are very abundant in both habitat types. Other secondary and tertiary consumers include various insects, reptiles, and mammals. There are relatively few mammalian carnivores on the islands, most of those formerly present having been exterminated (e.g., foxes, wolves, bobcats, pumas, bears). The terminal carnivore niches are filled mainly by predatory birds, the diamondback rattlesnake, and relatively inefficient mammalian predators such as raccoons and opossums.
Because of the complexity of food webs in forests and sloughs and the lack of local food habits data, no attempt will be made to trace specific nutrient path ways.
Fauna
NATIVE VERTEBRATES
Although this study produced much information on the distribution of vertebrates on the coastal islands, lack of adequate data on species interactions inhibits a detailed functional and conceptual discussion. However, an attempt has been made to integrate the available information on distribution, abundance, preferred habitat, and limiting factors. (See Appendices 2-5 for annotated lists of species, scientific names, and discussions of wading bird rookeries.)
Because of the limited size of the islands, population size is restricted and immigration is limited by open water barriers, restricting opportunities for genetic exchange. Such conditions favor the evolution of forms that are phenotypically distinguishable from their mainland ancestors. Island populations are frequently recognized as distinct taxonomic entities, and currently there are four recognized forms that are restricted primarily to one or more of Georgia's barrier islands: the Cumberland Island pocket gopher (Geomys cumberlandius), the Anastasia Island cotton mouse (Peromyscus gossypinus anastasae), the St. Simons Island raccoon (Procyonlotor litoreus), and the Blackbeard Island deer (Odocoileus virginianus nigribarbis). However, lack of specimens and historical data relating to the island fauna inhibits study of the relationships of populations.
1. Mammals: The mammal fauna of the Georgia islands has been poorly known, mainly because there has been little systematic collecting on the islands. Access to many of the islands has been restricted, and investigators sometimes have been denied permission to collect mammals on some of the privately owned islands (e.g., Bangs 1898; Elliot 1901).
Subsistence hunting, deliberate persecution, and disease probably caused extirpation of some species of mammals from the islands. Habitat changes effected by agriculture and timber management probably have not caused the loss from the islands of any mammal populations. However, it is evident that habitat changes associated with certain types of development may seriously reduce or eliminate some populations before their taxonomic position, range, distribution, and ecology can be determined. Therefore, during the course of this study, a concerted effort was made to develop a check list of existing mammals of the islands. The results are presented in Appendix 5 and are summarized in Table 8. The following discussion deals with select species and groups (see also sections on beach fauna, marsh fauna, and marine mammals).
The opossum is conspicuous on the islands where it occurs. The past and present distribution of the opossum suggest how man may have affected the distribution of island animals. White (1849) reported that the opossum was abundant on Cumberland Island, but subsequent visitors made no mention of its presence there. A plausible explanation for the opossum's absence was given by Lucy Ferguson (in Lee et al. 1963), a lifetime resident of the island, who stated that after the War Between the States the freed slaves killed for food all of the opossums on Cumberland and Little Cumberland islands. The present distribution of the opossum is confined to Sapelo, Little St. Simons, and islands to which man has constructed bridges and thus provided a route for invasion and reinvasion, i.e., Cockspur, Tybee, St. Simons, Sea, and Jekyll.
TABLE 8. Mammals of the coastal islands of Georgia.a
| Species | Oysterbed | Cockspur | Tybee | Little Tybee | Wasaw | Ossabaw | St. Catherines | Blackbeard | Sapelo | Wolf | Little St. Simons |
St. Simons | Sea | Jekyll | Little Cumberland |
Cumberland |
| Opossum | O | O | S | O | S | O | S | L* | L* | |||||||
| Short-tailed shrew | S | S | S | |||||||||||||
| Least shrew | S | S | S | |||||||||||||
| Eastern mole | S | S | S | S | S | S | O | S | ||||||||
| Southeastern myotis | S | |||||||||||||||
| Eastern pipistrelle | O | S | ||||||||||||||
| Big brown bat | S | S | ||||||||||||||
| Red bat | O | |||||||||||||||
| Seminole bat | S | S | ||||||||||||||
| Yellow bat | S | |||||||||||||||
| Cottontail rabbit | O | |||||||||||||||
| Marsh rabbit | S | S | O | O | S | S | S | S | S | O | S | S | S | S | S | |
| Gray squirrel | O | L | S | L | S | S | O | O | S | S | ||||||
| Fox squirrel | S | O | S | |||||||||||||
| Southern flying squirrel | O | |||||||||||||||
| Cumberland Is. pocket gopher | S | |||||||||||||||
| Marsh rice rat | S | S | S | S | S | S | O | S | O | S | ||||||
| Eastern harvest mouse | S | |||||||||||||||
| Oldfield mouse | L | |||||||||||||||
| Cotton mouse | S | S | S | S | S | S | ||||||||||
| Hispid cotton rat | S | S | S | S | S | S | S | O | S | |||||||
| Eastern wood rat | L | |||||||||||||||
| Black rat | S | S | S | L | S | S | S | S | ||||||||
| Norway rat | L | S | S | L | ||||||||||||
| House mouse | S | S | ||||||||||||||
| Nutria | L* | |||||||||||||||
| Goose-beaked whale | S | L | S | |||||||||||||
| Pygmy sperm whale | L | S | L | L | S | S | ||||||||||
| Dwarf sperm whale | S | |||||||||||||||
| Atlantic bottle-nosed dolphin | L | S | S | S | S | S | S | |||||||||
| False killer whale | L | |||||||||||||||
| Pilot whale | S | S | S | L | S | S | ||||||||||
| Gray fox | L* | |||||||||||||||
| Black bear | L* | L* | ||||||||||||||
| Raccoon | L | O | O | O | S | S | S | S | S | S | O | S | O | S | S | S |
| Mink | L | S | L | L | S | L | O | O | S | O | S | |||||
| River otter | L | L | L | L | L | L | S | L | L | L | L | L | L | S | L | |
| Bobcat | O* | S* | L* | L* | ||||||||||||
| Manatee | L | |||||||||||||||
| European wild boar | L* | O* | O* | O* | L* | O* | O* | |||||||||
| European fallow deer | L | O | O | O | O | |||||||||||
| Red deer or European elk | L* | |||||||||||||||
| White-tailed deer | L | L | S | S | L | S | S | L | L | L | L | L | L | S | ||
|
aSymbols—S=specimen. L=literature; O=observed; *=extirpated; Scientific names and documentation in Appendix 5. | ||||||||||||||||
Rodents are represented on the islands by gray and fox (introduced) squirrels, pocket gophers, and native and naturalized rats and mice.
Gray squirrels are absent on Blackbeard and Little Cumberland islands and recently have been introduced on Tybee, Wassaw, Ossabaw, and Sapelo islands (Tomkins 1965a). It is not known if those on Cumberland and St. Catherines are native. Hardwood forests are preferred habitat and high populations of squirrels often occur near residential areas.
The Cumberland Island pocket gopher described by Bangs (1898) is an insular mammal species occurring only on Cumberland Island. It is now endangered and should be added to the list of Rare and Endangered Fish and Wildlife of the United States (U.S. Fish and Wildlife Service 1968).
When Bangs first collected the species, he said, "... the hills straggled off through the pine woods for miles." E. B. Chamberlain of the Charleston Museum visited the island in 1932 and 1933, and his notes (Charleston Museum) indicated "several nice colonies on the island. Largest on golf links at Stafford Place." During a recent collecting expedition (March 1970), Baker and Dopson found that only one old field exhibited signs of gopher activity. A month later, this field was plowed for agriculture.
The drastic reduction in the pocket gopher population on Cumberland probably has been due to agriculture, excessive grazing, and, to some extent, excessive specimen collection. There are at least 53 specimens of this species in museums, three of which were pregnant with a total of six embryos. Additional collecting should not be permitted.
The habitat of this species is similar to that of mainland forms in that relatively loose friable soil is preferred. Specimens from Cumberland have been taken from old fields and pine woods. Harper (1912) stated that Cumberland was the only island with longleaf pine and turkey oak and that the presence of the pocket gopher might somehow be related to this. However, these trees do occur on some of the other islands, and the most recent (March 1970) pocket gopher sign observed during this study was 4-5 miles from the nearest longleaf pine stand.
Rats and mice are found on the islands, but the species involved and their distributions are poorly known. The cotton rat is found on most of the islands, living preferentially in old fields and fence rows but also in the dunes and salt marshes. Cotton rat remains is one of the most common items occurring in pellets regurgitated by barn owls on the islands. A large collection of owl pellets from Sapelo yielded 152 cotton rat skulls and 26 skulls of three other mammal species.
The cotton mouse is abundant on only two islands, Cumberland and Little Cumberland, although it is present on several others. The Anastasia Island cotton mouse, described from specimens from Anastasia Island, Fla., occurs on Little Cumberland and Cumberland islands. The population on Anastasia Island has been drastically reduced or extirpated (Pournelle and Barrington 1953), so the populations on Little Cumberland and Cumberland islands should be protected to insure survival of this insular subspecies. This subspecies should be considered for inclusion in the list of rare and endangered wildlife, previously mentioned (U.S. Fish and Wildlife Service 1968).
The reason for the scarcity of native mice on most of the islands is a matter of some conjecture. Pournelle and Barrington (1953) suggested, among other reasons, possible competition between the cotton mouse and cotton rat. They reported that an inverse relationship appeared to exist between populations of cotton rats and cotton mice. Data obtained from trapping and owl pellets suggest that this relationship holds on Tybee, Sapelo, Jekyll, Little Cumberland, and Cumberland islands.
The distribution of the house mouse (unintentionally introduced) is peculiar in that, while normally almost ubiquitous, it is restricted on the coast to two islands near Savannah. On Little Cumberland and Cumberland islands, the habitat normally occupied by house mice is occupied by cotton mice. On other islands, such as Sapelo, no sizeable numbers of any kind of mouse have invaded what appears to be favorable habitat.
Carnivores on the islands formerly included several species now extirpated. Today, only the raccoon, mink, and otter represent this group. Mink and otter are discussed in the section on marshes. Raccoons are very common. They inhabit every island and occur in every habitat type. Populations are often high, and several wildlife refuges conduct control measures primarily during the sea turtle nesting season. However, control is only temporary because of rapid recruitment from surrounding areas. Raccoons are hunted by some island inhabitants, but raccoon hunting has declined in recent years. Rabies occurs with a relatively high frequency in raccoon populations on the mainland, but it has not been reported from raccoons on the coastal islands.
The white-tailed deer is the only native hoofed animal on the islands. The introduced fallow deer is discussed later. White-tailed deer now occur on most of the barrier islands although many existing populations are not derived entirely from native stock. Unfortunately, deer that have been used to stock and restock the islands often were not obtained from nearby islands having native white-tails. For instance, deer from Pennsylvania were introduced on Wassaw Island (Francisco et al. 1970). The past introductions of deer having diverse genetic backgrounds confuses the exact taxonomic status of the herds occurring on some of the islands. The subspecies Odocoileus virginianus nigribarbis occurs on Blackbeard and Sapelo islands and apparently, at least on Blackbeard, has not been genetically contaminated by introduction of animals from other geographic areas. The range of O. v. virginianus which includes the Georgia coast is poorly known; Hall and Kelson (1959), however, include Cumberland Island in the range of this subspecies.
The island deer occupy almost all of the terrestrial habitat types. They are most conspicuous in the live oak-pine forest but are frequently seen in the dune areas. Deer are also sometimes observed on the island edge of the salt marsh.
The white-tailed deer that occurs on the islands generally is smaller in size than the deer that occurs on the adjacent mainland. Deer from Blackbeard Island average 60 lb (L. Wineland in Lund et al. 1962). Mature bucks weigh about 110 lb. The small size of island deer may be a genetic adaptation or a response to habitat deficiencies or both. Annual archery deer hunts are held on Blackbeard Island. Deer hunting is conducted on some other islands by island owners; generally the harvest is low.
2. Birds: The diverse habitat of the barrier islands supports a large number of bird species. Appendix 3 includes a list of species, seasonal abundance, and habitat preferences. The extensive live oak forests draped with festoons of Spanish moss provide nesting and feeding habitat for large numbers of songbirds and woodpeckers. Pastures and fields are used by wild turkeys, cattle egrets, meadowlarks, and other ground-feeding birds. Sloughs and ponds are favorite feeding areas for anhinga, ibis, herons, egrets, and waterfowl. Large wading bird rookeries are also located near many of the sloughs and ponds on some of the islands.
The live oak and pine forests are used extensively as feeding areas by many resident species. Resident birds that occupy this habitat type include Carolina chickadee, brown-headed nuthatch, Carolina wren, brown thrasher, pine warbler, cardinal, and rufous-sided towhee (Teal 1959). Numbers of birds in the live oak-pine forests are greatly augmented each year with migrant species. Some migrants such as the yellow-bellied sapsucker, ruby-crowned kinglet, eastern phoebe, and white-throated sparrow become winter residents. Other migrants that feed in oak-pine forests include solitary vireo, black-and-white warbler, prothonotary warbler, and American redstart (Teal 1959).
The habitat diversity provided by the live oak forests will become in future years increasingly important for many bird species as mainland live oak forests are reduced in extent by modern pine forestry practices and industrial, residential, and recreational development. Preservation of the live oak forests on the islands should be continued as in the past.
The distribution of the endangered red-cockaded woodpecker (U.S. Fish and Wildlife Service 1968) on the barrier islands is unknown. Burleigh (1958) stated that this bird has been reported breeding on Blackbeard Island, but it is not known to breed there now. Teal (1959) reported the species on Sapelo Island but stated that it was rare. Its distribution apparently is determined by the presence of mature pine trees infected with red-heart disease (Fomes pini). Red-cockaded woodpecker nests are restricted to such trees. Tracts of pinelands containing known nesting sites of red-cockaded woodpeckers are being preserved on the mainland.
The blue jay is a common mainland bird that has a sporadic distribution among the islands. Its presence on the mainland and on some islands has been described or reported by several observers (Austin 1965; Davenport 1966; Tomkins 1965a). Tomkins (1965a) reported that the blue jay was present on Tybee, St. Simons, and Jekyll islands. Sprunt (1936) reported it on Cumberland Island. Jays were not reported by Tomkins on some of the barrier islands such as Sapelo, Blackbeard, Wassaw, and Little Cumberland. Since Tomkins' paper, the blue jay has been reported on Sapelo (1966) and is now a common permanent resident there. Blue jays were not seen during recent trips to Ossabaw Island (June 1970) and Blackbeard Island (June 1971). Apparently the distribution of the blue jay is influenced by human habitation. The jay is very common on Jekyll and St. Simons islands where high-density development has occurred.
Robert et al. (1956) reported the absence of the tufted titmouse from Sapelo and other islands. The Carolina chickadee, a frequent associate of the tufted titmouse on the mainland, is rather common on Sapelo Island.
Several of the islands have small wild turkey populations. The wild turkey probably was extirpated from most of the islands and later reintroduced. The present populations are relatively tame and do not exhibit typical behavioral characteristics of wild turkeys. Blackbeard Island probably has the purest strain of wild turkey, but the population currently is very low. Turkeys could be reintroduced on those islands having few or no turkeys, but every attempt should be made to obtain live-trapped wild stock for restocking. The Georgia Game and Fish Commission has begun a wild turkey management program on the R. J. Reynolds Wildlife Refuge on Sapelo Island. Eventually the Commission hopes to remove a number of turkeys annually for restocking elsewhere in Georgia.
Five wading bird rookeries occur on the barrier islands. Two are located on Ossabaw Island, one on Blackbeard Island, and two on Cumberland Island. Species that nest in these rookeries include snowy, cattle, and common egrets; and black-crowned night, great blue, Louisiana, and green herons. Wading bird rookeries are mapped and described in Appendix 4.
The maintenance of rookery sites is essential in the conservation of wading birds. Rookeries are usually used year after year when not encroached upon by development, pond drainage, or excessive disturbances. Rookeries on the islands are perhaps the ones best protected from human disturbance. However, some rookeries are lost by habitat changes such as have occurred at the rookery on the north end of Sapelo. This rookery site was formed when a finger of salt marsh was diked and then flooded by an artesian well. The rookery was abandoned after aquatic vegetation became too dense.
The ponds and sloughs on the islands are intensively used by waterfowl during the fall and winter. Blackbeard Island National Wildlife Refuge has management units consisting of permanent ponds. Ducks wintering on Blackbeard include, in order of abundance, ring-necks, scaups, mallards, gadwalis, baldpates, canvasbacks, pintails, green-winged teals, and shovelers. Duck populations have ranged from 10,000 during 1955-56 to 61,660 in 1960-61 (U.S. Fish and Wildlife Service 1962). Most of these species of ducks use the ponds and sloughs on other islands. The proximity of the Blackbeard refuge positively influences the waterfowl populations on the other islands. (Waterfowl are discussed in more detail in the chapter on marshes.)
Many other water birds are attracted to the aquatic habitat on the islands. Most common among these are the coot and both common and purple gallinules. Wood ducks also breed and raise broods in the sloughs and ponds. Nesting boxes have been erected on Blackbeard Island in an attempt to increase local populations of wood ducks.
The endangered southern bald eagle (U.S. Fish and Wildlife Service 1968) occurs uncommonly on the Georgia coast. The size of the present resident population is not known. One pair nested and reared two young on St. Catherines Island during 1970. This is the only known reproduction occurring in coastal Georgia during 1970. Formerly the bald eagle nested on most of the barrier islands and elsewhere along the coast. The old-growth, dead timber that occurs on the islands provides preferred nesting habitat for bald eagles and ospreys. The lack of human disturbance also contributes to successful nesting. Great horned owls often nest in abandoned osprey and eagle nests.
3. Amphibians and reptiles: The herpetology of Georgia's barrier islands is poorly known. Until recently, many of the islands were inaccessible to herpetologists, and there has been very little collecting on the islands. Only one report has dealt exclusively with the herpetofauna of the islands: Martof (1963) listed the reptiles and amphibians collected on Sapelo Island during a 10-year period. The U.S. Fish and Wildlife Service has inventoried the larger vertebrates of the national wildlife refuges on the Georgia coast. Their reports list and discuss such forms as alligators and sea turtles (Blackbeard Island) but do not include the small reptiles and amphibians of the area. Neill (1948) discussed the island glass lizards collected on Wolf Island, a marsh island south of Doboy Sound.
Because there has been very little systematic collecting on the islands, a description and discussion of the herpetofauna of the islands still must be incomplete. An attempt has been made to collect specimen records, literature references, and reliable sight records relating to the amphibians and reptiles of the barrier islands. The resulting information is presented in Appendix 2. Undoubtedly, additions will be made to the list of species as the islands become more accessible to collectors.
A wide variety of habitats exist on the islands, but because of the restricted area of the islands some habitat types are necessarily limited (e.g., extensive swamps do not exist). Therefore, the occurrence of some reptiles and amphibians is restricted.
The herpetofauna of the barrier islands includes 2 salamanders, 11 frogs, 6 turtles (exclusive of one estuarine and three marine species), 1 crocodiian, 9 lizards, and 12 snakes. None of the species is endemic to the islands, although future taxonomic investigation may reveal subspecific differences among several species. Martof (1963) stated that all of the herpetofauna of Sapelo Island has been acquired from the adjacent mainland. This apparently is true for the populations occurring on the other barrier islands.
The extensive salt marshes and sounds which separate the islands from the mainland and from each other apparently present a rigorous barrier to most amphibians and many reptiles for colonization of the islands. The tidal amplitude (about 7 ft) is the greatest anywhere along the coast of the southeastern United States and estuarine salinites are high along the Georgia coast, ranging from 20 to 30 parts per thousand (ppt) (Williams 1964). The natural occurrence of most of the reptiles and amphibians on the islands is related to their (1) tolerance to brackish and salt water, (2) habitat preferences, and (3) locomotor abilities. Obviously some forms are better adapted than others to movement across the marshes and estuaries. Reptiles are protected by a tough skin and shell (turtles) or skin and scales (snakes and lizards), enabling them to better withstand the rigors of crossing the barriers to the islands. To some degree, this is reflected by the larger number of reptiles than amphibians that occur on the Georgia islands.
All of the frogs and toads occurring on the Georgia islands (except the spadefoot toad) also have been reported in or near brackish or salt water elsewhere (Neil 1958). For example, narrow-mouthed toads have been reported from brackish ponds, and Neill (1958) reported their occurrence on a salt flat at Merritt Island, Fla. Hardy (1953) observed that this amphibian appeared to have a "vigorous proclivity for haline habitats" in the Chesapeake Bay marshes. W. E. Brode (Neil 1958) found narrow-mouthed toads breeding near Bay St. Louis, Miss., in water too salty to drink. Similar tolerance to salt water has been reported for the southern leopard frog (Carr 1940; Pearse 1911) and the southern toad (Allen 1932; Neill 1958), so it is not surprising that the above species commonly occur on the Georgia barrier islands. Similar examples of adaptation to haline habitats exist for many turtles and snakes.
Certain habitat preferences have undoubtedly contributed to successful island colonization by some reptiles and amphibians. Engels (1949) reported he collected the southeastern five-lined skink on Harkers Island and Shackleford Banks, N.C., "beneath loose bark on standing trees." He suggested that this habitat preference may have favored the accidental transportation of the species to the islands. This skink occurs on Sapelo Island along with the five-lined and broad-headed skink.
The cottonmouth and the diamondback rattlesnake occur in considerable numbers on most of the islands. Carr (1936), Allen and Neill (1950), and Wharton (1969) have reported that cottonmouths occur on coastal islands in Florida. Neill (1958) reported that the diamondback rattlesnake is more common in the salt marsh than in any other habitat in two Gulf coastal counties in Florida. He also stated that it is a good swimmer and has been sighted in the ocean several miles from land.
The cottonmouth is semiaquatic but is often found considerable distances from water. Its preferred habitat includes the sloughs and ponds. It commonly feeds on aquatic vertebrates.
The diamondback rattlesnake occupies most types of terrestrial habitat on the islands. It may be found from the drift line along the beaches to the salt marsh. It is least common in the live oak forest. It is commonly thought that this snake is less abundant on islands having feral hog populations. Hogs are generally thought to eat snakes but data are lacking to support this supposition. If hogs do influence rattlesnake numbers, they may do so indirectly by altering the habitat of various prey species such as cotton rats.
Black racers, king snakes, and banded water snakes are perhaps the most common snakes on Sapelo Island. Black racers also are common on St. Catherines, Little Cumberland, and Cumberland islands.
The island glass lizard occurs on coastal islands in South Carolina, Georgia (Sapelo, Wolf), and Florida but is not an insular species. It also is found in the "scrub" patches in the interior of peninsular Florida (Neill 1958) and is known to occur in the vicinity of the Okefenokee swamp in Georgia (Wright and Funkhouser 1915).
Martof (1963) reported the green anole as the most common lizard on Sapelo Island. The large broad-headed skink and the diminutive ground skink are also common there, although the latter is seldom seen without searching under leaf litter.
The endangered American alligator (U.S. Fish and Wildlife Service 1968) occurs on all of the undeveloped barrier islands of Georgia and in portions of the marshlands. The Bureau of Sport Fisheries and Wildlife estimates the 1969 alligator population on their coastal refuges as follows: Savannah, 150+; Wassaw, few; Harris Neck, 10; and Blackbeard, 750-1000 (W. L. Towns, pers. comm.). Population estimates are not available for other barrier islands. The number of alligators on each island is constantly changing because alligators frequently move between the marshes and islands. Although the alligator is rigidly protected by state law in Georgia, some poaching undoubtedly occurs, mainly on those alligators inhabiting the marshes. Alligators on the islands are afforded additional protection by the island owners.
The alligators in the coastal area of Georgia contribute significantly to the total numbers of this species. The barrier islands are, in a way, individual refuges for the alligators, and they play an important role in the conservation of this interesting reptile.
The amphibians (13 species) have not been as successful as the reptiles (28 species, exclusive of estuarine and marine forms) in island colonization. The frogs and toads have been more successful than the salamanders. Only two salamanders, both aquatic, have been reported or collected on the islands. The amphiuma or congo eel, a large aquatic salamander, is probably common in the freshwater sloughs and ditches on many of the islands. However, it is difficult to collect and little is known of its distribution; several have been collected on Sapelo Island. Similar aquatic salamanders, such as the siren, have not been reported from the barrier islands. The central newt, also aquatic, is fairly common on Sapelo Island. This salamander has an immature terrestrial stage called an eft which is commonly found under logs. The eft is especially susceptible to predation by feral hogs on some islands. This may influence the distribution or abundance of this species.
The pig frog was reported by Martof (1963) to be the most common frog or toad on Sapelo. However, pig frogs are conspicuous, and it is difficult to compare their population density with less conspicuous forms such as the tree frogs.
INTRODUCED VERTEBRATES
1. Exotic birds and mammals: Several exotic birds and mammals have been intentionally introduced onto the barrier islands. Only a few have become established as perpetuating populations. The relatively large number of species that have been introduced on the Golden Isles is related to the affluence of the island owners. Many owners were attempting to supplement the native wildlife populations with game species that might increase hunting diversity.
Norris (1956) reported that five species of gallinaceous birds were introduced onto Jekyll and Sapelo islands between 1890 and 1924. These included the guinea fowl (Numida goleta), tinamou (Tinamus robustus), Central American currasow (Crax rubra), ocellated turkey (Agriocharis ocellata), and chachalaca (Ortalis vetula). Since all of these birds are primarily forest-dwelling species, it was hoped that they would thrive in the oak-pine forests of the islands. However, only the chachalaca became established—on Sapelo Island where a small population has maintained itself since 1923. The chachalaca is known to inhabit the dense stands of live oaks on Sapelo (Jenkins 1949), but little else is known of its ecology and relationships to other species on the island.
Several species of big-game animals have been introduced on the islands. These include European wild boar (Cumberland, Little Cumberland, Jekyll, St. Simons, Little St. Simons, Ossabaw, Wassaw); fallow deer (Jekyll, Little St. Simons, Sea, St. Simons); and red deer (Little St. Simons). Only the fallow deer remains. A large population (400-500 animals) exists on Little St. Simons Island, and a small number occurs on Jekyll Island; individuals occasionally move from Little St. Simons to Sea and St. Simons islands. Although the ecological impact of these animals on the island flora has not been studied, casual observation indicates they contribute to the severe overbrowsing of the vegetation on Little St. Simons, including the dune vegetation and some marsh vegetation.
Hanson and Karstad (1959) said that the European wild boar was introduced into feral herds of swine to increase their sporting value. However, the European wild boar has been extirpated from those islands where it was introduced, or it has been genetically integrated into the large feral hog populations that occur on many of the islands. The ecological impact of feral hog populations will be discussed in the following section.
The nutria, introduced on Blackbeard Island by the U.S. Fish and Wildlife Service in 1949 (Jenkins 1953) as a possible biological control agent for several species of aquatic plants, failed to control or eliminate the problem plants and was exterminated by the early 1960s. A few nutria are reported to occur in the coastal marsh near Brunswick (Wilson 1968).
The fox squirrel probably was introduced on Ossabaw Island in the 1920s or 1930s. Although the exact origin of the squirrels is not known, they are thought to have been obtained from Michigan (Charles Marshall in corresp. 13 October 1970). The ratio of fox squirrels to gray squirrels is approximately 20:1 on Ossabaw (Charles Marshall in corresp. 13 October 1970).
2. Domestic and feral animals: Several species of domestic animals have been introduced on the islands. These include common household pets such as dogs and cats and farm animals such as horses, donkeys, cows, hogs, goats, and chickens. (See Table 9 for distribution.) Some of these animals have become established as feral populations. Many of the early owners of the islands derived a considerable portion of their income from the sale of cattle and hogs raised on their islands. However, recent owners have had other sources of income and the need for good animal husbandry has declined. To an extent, this has created ecological problems for many of the islands.
Large, free-ranging herbivores such as horses and cattle and the omnivorous pig have seriously reduced the extent and quality of several island habitat types. The most serious damage has occurred on the dunes. Dune-stabilizing plants have been eliminated or seriously reduced on some islands. The importance of these plants in dune stabilization, hence island stabilization, has been discussed in a previous section.
The mere presence of livestock does not necessarily constitute a threat to the habitat. Only when the numbers of these animals become too great does serious damage occur. In some instances cattle may actually constitute a desirable ecological element. For instance, in the absence of fire, cattle create and maintain openings within the island interior compatible with wild turkey management. Stoddard (1963) cited the attractiveness of grazed areas to turkeys during the brood-rearing season. Recent studies in Alabama (Hillestad and Speake 1970) revealed the apparent compatibility of cattle and wild turkeys. The cattle maintained brood-rearing habitat (pastures) that provided poults with forage and an abundant supply of easily obtainable insects—a preferred food of turkey poults. Prescribed numbers of cattle could enhance turkey production in some habitat types.
TABLE 9. Present distribution of domestic and feral mammals on the barrier islands.
| Species | Island |
| Hog | Cumberland, Little Cumberland, Jekyll, St. Simons, Sapelo, St. Catherines, Ossabaw, Wassaw |
| Horse | Cumberland, St. Simons, Sea, Little St. Simons, Sapelo, Ossaba |
| Donkey | Cumberland, Ossabaw |
| Cattle | Cumberland, St. Simons, Little St. Simons, Sapelo, St. Catherines, Ossabaw |
| Goat | Ossabaw |
Although there is a certain amount of overlap of food habits between free-ranging cattle and deer, it is not significant enough to force the exclusion of one species. Moderate numbers of both animals may be compatibly maintained on the islands.
Cattle also may be used to maintain open, park-like conditions under the live oak forest where these conditions cannot be obtained satisfactorily with fire. Such conditions facilitate travel and observation and generally enhance the recreational value of this habitat type.
Several of the islands have large populations of free-ranging hogs. On many of the islands they are in a semi-wild or feral state. Most writers are in agreement that free-ranging hogs seriously compete for food with many desirable wildlife species, including deer and turkeys. Many people who are familiar with the hog populations on the islands contend that they interfere with reproduction of ground-nesting birds such as quail and turkey. Hogs also may be influencing small vertebrate populations on some islands. The serious problem of hog depredation on sea turtle nests is discussed in another section.
C. W. Wharton (pers. comm.) found that hogs on Cumberland were feeding in the dune areas on pennywort, a plant which assists in dune stabilization.
Undoubtedly, numbers of free-ranging hogs on several of the islands should be reduced until their ecological relationship to island fauna and flora can be better evaluated.
Bantam chickens recently have been introduced on Ossabaw Island. These domestic birds are free-ranging and are subjects of a study of the ecology of domestication (I. L. Brisbin, pers. comm.). A pure wild strain of red jungle fowl (Gallus gallus) is to be released on Ossabaw in a continuation of these studies. Since chickens and jungle fowl are potential vectors of blackhead (Histomonas meleagridis), a deadly disease of wild turkeys, precautions have been taken to insure against accidentally introducing it into the turkey population on Ossabaw.
INVERTEBRATES—NOXIOUS ARTHROPODS
The diversity of the invertebrates of the island interior is too complex and inadequately studied to permit a comprehensive discussion here. Therefore, only those forms creating human discomfort and annoyance are included in the following discussion. Mosquitoes, sandflies, deerflies, chiggers, and ticks appear in vast numbers periodically and cause considerable human discomfort as well as serving as potential vectors of a number of diseases affecting man and domestic animals. Recreational activities on the island must sometimes be asynchronous with the appearance of these forms.
Portions of the salt marshes are the breeding sites of two mosquitoes, Aedes sollicitans and Aedes taeniorhynchus, which often are a severe annoyance to people inhabiting the islands (salt marsh mosquitoes are discussed in Chapter 4). Domestic mosquitoes, including Aedes aegypti and Culex pipiens quinquefasciatus, are common around dwellings on the islands. The Culex mosquito breeds in ground pools and in polluted waters. Aedes aegypti is a container-breeder and often can be controlled by eliminating breeding sites such as trash dumps containing cans and bottles (King et al. 1960).
Perhaps the most annoying insects to man on the islands are the deerflies of the family Tabanidae and principally of the genus Chrysops. These diurnal insects are most common in wooded areas on the islands and often emerge in such numbers that deer and cattle move into the dune areas where the insects are less abundant and where the wind retards the flight of the flies.
Several species of ticks occur on the islands. The more common species include Ixodes affinis, I. scapularis, Dermacenter variabilis, Amblyomma americanum, and Haemaphysalis sp. White-tailed deer commonly are heavily infested with Amblyomma americanum. Ixodes scapularis has been found on both warm blooded and cold-blooded hosts. All of these species occasionally are an annoyance to people on the islands.
| <<< Previous | <<< Contents >>> | Next >>> |
chap3.htm
Last Updated: 1-Apr-2005