|
NATIONAL PARK SERVICE
The Impact of Three Exotic Plant Species on a Potomac Island |

|
CHAPTER 4:
DISCUSSION
Lonicera japonica
It appears that the impact of Lonicera japonica upon the indigenous vegetation of Theodore Roosevelt Island is to destroy the forest, and the key to this destruction is the amount of light that reaches the forest floor.
From general observations of the past (qualitative survey, if you will) both on and off the island, it would appear that L. japonica does not do well in the shade. Similar observations have been made and similar conclusions have been drawn by other observers including Leatherman (1955:26, 27, 58). Everyone has a hunch, but there is a dearth of data. Good quantitative data to support this hypothesis are lacking in the literature. Leatherman's study comes closest and a review and commentary on that study will be instructive. Leatherman transplanted 10 cuttings to each of three different habitats (open, deciduous forest, evergreen forest) at each of four elevations (Leatherman 1955:19-21). After 3 months, a comparison was made; the best growth and cutting survival occurred in the open habitats (at 6000 ft and 5200 ft), but the poorest growth and survival also occurred in open habitats (at 3500 ft and 1500 ft). Slight differences in shoot-root ratios (dry-weight basis) of L. japonica in the different habitats were not statistically significant (Leatherman 1955:21). These habitats were described (Leatherman 1955:22, 23) but the description of light intensity was taken from a measurement on only one day. In studying the development of the seedling, Leatherman (1955:40, 43, 44) grew 10 plants each in the greenhouse; full sun, 25% of full sun, and 5% of full sun. The average weight (in mg) of the combined shoots and roots is 0.4 mg higher in the 25% of full sun than in full sun (Leatherman 1955:43; my calculation from Leatherman's data). The 5% of full sun was 9.5 mg lower than the 25% of full sun (my calculation). The average length of shoot, however, did decrease with full sun, while the average length of root increased. From colonies of mature Lonicera japonica, Leatherman (1955:47) found the average weight of 100 sun leaves to be 0.063 g. while that of shade leaves was 0.031 g. Three photosynthesis measurements were also made on leaves by using a modification of the matched-leaf method of Denny: potted plants, cuttings, and intact plants (colonies) (Leatherman 1955:48, 49). In each case, the dry-weight increase in full sun and 25% of full sun was determined, and for the cuttings the dry-weight increase for 5% of full sun was also determined. The percent of dry-weight increase was greatest in full sun and least in 5% of full sun; this was significant at the 5% level (Leatherman 1955:49, 50). Full-sun leaves consistently showed greater weight than the 25% of full-sun leaves; however, the differences were not statistically significant (Leatherman 1955:49, 50). In another study of light, Leatherman (1955:50-53) placed 10 cuttings each in five plots near the greenhouse; varying layers of cheesecloth were placed over four of the plots. The experiment lasted 160 days. Actually, more plants survived under 50% and 25% of full sun than under full sun. The number of nodes per plant decreased with decrease in light. The combined average dry weight of shoots and roots declined consistently from full sun to 5% of full sun.
Thus, it would appear that there are some quantitative data which are not altogether consistent in their support of light as a limiting-factor hypothesis for Lonicera japonica in the deciduous forest. By using intact plants already and long established in the habitat, I avoided the variation that would be associated with survival of cuttings. Instead of taking only one light measurement in a habitat, I replicated the measurements both by location and time in each habitat. In addition, I replicated my cheese cloth-shading experiments.
The sequence of events that leads to forest destruction by L. japonica appears to be as follows. To begin with, some disturbance of the vertical structure of the forest must occur, for L. japonica areas have a different proportion of the vertical layers present than the nonexotic forest (Table 78; also Tables 76, 77). It is not necessary that the overstory be removed to bring about this change (Table 81). The Acer negundo which was removed from Little Island with subsequent invasion by L. japonica was probably understory. As shown in Table 81, it is the shrub layer which is the most variable between the Lonicera areas and the forest without exotics.
The disturbance of the vertical structure allows more light to come into the forest. This is shown by increased light in the L. japonica areas compared with the forest without exotics (Tables 51 through 58). (Recall that both vertical structure and light intensity were studied at the same stations.) In the winter (December, February, March) the least-disturbed L. japonica areas have similar light intensities to the forest without exotics. This is a time when Quercus spp. and Fagus grandifolia (beech) would be expected to have fewest leaves. The only other time of similar light intensities is in June.
The more light, the heavier the growth of Lonicera japonica (Tables 67, 75). This light-stimulated growth is in the form of cover (vigor) as well as biomass (Table 75). With changing degrees of shade, the biomass of chlorophyll does not change at a rate different from that for leaf biomass as a whole. Growth may be slightly faster on a new area than on an area already established with L. japonica due to less competition.
In a forest which is less disturbed, this growth of L. japonica on the forest floor suppresses the normal ground layer and replaces it with another ground layer. The new ground layer is, in a sense, not a replacement but an additional layer formed at a lower height than the original (Table 77). In a badly disturbed forest, the L. japonica becomes vigorous enough to stand at the same height as the original ground layer (Table 77).
This differential growth is reflected in the amount of biomass produced (Tables 2, 6), with significantly more being produced in the habitat with the greater vertical-structure disturbance. In the slightly disturbed area, growth of Lonicera japonica is relatively slow, with no significant difference between the biomass growth of 1 year and that of 1.25 years (Table 5). Frequency as a substitute for cover reflects the same growth rate (Tables 9, 10). In a more disturbed area, production of biomass is more rapid (Table 5). By using frequency as an indication of cover, it will be seen that in highly disturbed areas 90 days after removal, L. japonica will appear as though it had not been removed (Table 15). Cover recovery is not so rapid in areas disturbed only slightly (Tables 10, 17); however, after 1.25 years, recovery may approach the original condition (Table 10).
Some of the impact has been alluded to in the suppression of the ground layer. Since plants of all forest layers must pass through this ground layer in their growth, suppression in this layer will promote far-reaching structural changes over a period of generations. Life form or growth form is not synonymous with vegetational stratum, but there is a relationship between the two because of the usual heights attained by the different growth forms.
In mildly disturbed forests, Lonicera japonica suppresses trees, particularly Prunus serotina, and other woody plants, especially Parthenocissus quinquefolia (Table 30). The data show no significant difference between the impact of L. japonica on herbs and on woody plants (Tables 30, 31). The anomalous herb data of Table 30 are very close to meeting the statistical significance level.
When the forest is highly disturbed, suppression becomes more marked (Table 35). Although herbs in general and Oxalis stricta in particular are suppressed by this exotic vine, one species, Allium vineale, is not so affected. Apparently, the long, narrow leaves of this exotic herb which poke up through the Lonicera japonica get sufficient light and nutrients to remain unaffected by the presence of the vine. Herbs are more abundant in open areas than in forests and the highly disturbed forest is like an open area. This explains the greater suppression of herbs here than in the mildly disturbed forest (Table 38), although the suppression is not statistically significant.
The only species which occurred frequently enough in both the mildly and highly disturbed forests to permit analysis was Parthenocissus quinquefolia. This woody vine was suppressed more in the mildly disturbed habitat than in the highly disturbed habitat (Tables 30, 35, 37). The nontree, woody plants as a group are not suppressed in this highly disturbed area (Table 35); these are mostly woody vines, not shrubs. Under more natural conditions, these vines receive sunlight in the tops of the trees. In this highly disturbed habitat, they receive the light on the ground (Tables 51-58). Regardless of the degree of disturbance, Lonicera japonica definitely suppresses trees (Tables 30, 35). In the highly disturbed forest, Liriodendron tulipifera and Ulmus americana were particularly affected. These results agree with the report of Little and Somes who say (1967:1) that the vine is particularly luxuriant in openings and usually prevents reproduction of other vegetation. However, they present no data in support of their statement.
The impact of Lonicera japonica in a highly disturbed forest is apparently not different in kind from that of a mildly disturbed forest (Tables 38-41). Therefore, the impact of this exotic vine in the highly disturbed forest is merely a magnification or speeded-up process of the same impact on a mildly disturbed forest. The three tree species (Ulmus americana, Prunus serotina, Liriodendron tulipifera) which are most adversely affected by L. japonica are among the dominants of the island upland (see Introduction). With reproduction in all potential layers suppressed, the vertical structure will become more open or empty as the mature vegetation dies. This will allow more light to come in and speed up the growth of L. japonica, which will hasten the demise of the forest. At the same time that the forest is dying, the species composition will change because all species are not affected at the same rate. At present, the forest is slowly headed toward one without U. americana, P. serotina, and L. tulipifera as principal dominants.
If the present infestation on healthy trees were the same as on dying trees and this in turn were the same as on dead standing trees and likewise on dead down trees, it would be assumed that the infestation was stabilized. The principal present overstory dominant, Ulmus americana, is relatively free of Lonicera japonica (Table 82). The low infestation associated with dead standing Ulmus is approximately the same as that of all other dead standing overstory trees (Table 83). The dying trees other than Ulmus have essentially the same infestation level as the dead trees, but Ulmus has significantly more L. japonica than other dying overstory trees (Tables 83, 84). Something has stimulated L. japonica in the recent past to grow on more trees of U. americana. The sequence appears to be as follows. In 1963, during the time when observations were being made for the dendrological survey and floral checklist, several Ulmus (approximately 50-73 cm diameter, 17-75 cm above soil line) were cut ostensibly for control of Dutch elm disease. However, neither trees nor bark were removed from the island. This increased the breeding habitat for the inner-bark boring beetle Scolytus multistriatus (Marsh.), the smaller European engraver (Anderson 1964:239; Welch and Matthysse 1960:4, 5, 9; Boyce 1948:299). I have not seen the galleries of the native elm bark beetle Hylurgopinus rufipes (Eich.) on the island. Both fungus and beetle build up in these dead logs (Matthysse 1959:2, 6, 9). It usually takes a large number of feeding marks by the beetles to cause infection (Matthysse 1959:10). With an increased population of both fungus and beetle, more Ulmus would have the disease. By 1965, the incidence of the disease on Theodore Roosevelt Island reached the point that plant pathologist Horace V. Wester (National Park Service) recommended an active sanitation program. but such a program was not carried out (Chick 1966). As the trees die from the disease, the crowns open up and allow more light to enter the forest. Lonicera is very responsive to light (Table 75), especially when invading an area (Table 67). Thus, the proportion of U. americana trees with L. japonica on them increases over the standing dead trees which represent the infestation of a previous generation of living trees (Table 83). At the same time that the exotic vine was stimulated to grow on an increasing proportion of dying U. americana, the surrounding vegetation was also stimulated and the crowns of the nearby trees expanded. The beetles can breed only in tight-barked wood (Welch and Matthysse 1960: 6, 9). By October 1972. there was already no breeding habitat on the down trees (Table 96). With the beetle population down, the death rate of Ulmus decreased and hence the invasion rate by L.japonica would also decrease so that the present proportion of infestation on the current generation of healthy trees has decreased (Tables 82, 83). But it is not down to the 8 or 9% found on dead standing trees (Tables 83, 84), which is greater than the 3% growing on trees before they fell (Table 85). Overall, the conditions for growth of L. japonica have improved over the years.
The growth of Lonicera japonica on overstory elms serves to some extent as an indicator of the vigor of the tree, but, except for some root competition, probably does not affect that vigor. Reports of L. japonica overwhelming vegetation (Whipple and Moeck 1968:1; Little and Somes 1967:1; Penfound 1966:190, 191; Gunning 1964; Oosting 1956:208; Leatherman 1955:26, 27, 84, 86; Kephart 1939:1; Handley 1945:263) are with less than usual-sized overstory trees. Daubenmire (1965:304, 305) points out that woody twiners constrict the host stem and subsequently interfere with downward translocation in the host and the host tissue overgrows the liana with apparently similar results. Leatherman also suggests (1955:26, 27) that death or impaired growth of trees was due to interruption of translocation of food to the roots by L. japonica. Most overstory-sized trees escape this kind of death or impairment. Since vines have a weak stem, twiners apparently need branches or stubs for support as they climb. Honeysuckle stems may elongate up to about 1.5 m in a growing season (Leatherman 1955:45, 68). On relatively low-growing vegetation, there are many small branches on the trunks for the vine to rest upon. Large trees have no such supports unless another vine with branches is already on the tree. Lonicera could not grow up with the tree and thus have its leaves in the forest overstory like Vitis spp. because it overwhelms and suppresses small trees. When an overstory tree falls, whether it falls naturally or is cut, the opened canopy with increased light promotes the growth of the vine (Table 85).
Honeysuckle is able to outcompete the native deciduous vegetation in the lower stratal layers (and hence eventually the upper stratal layers) apparently because it is evergreen in this area (see also Leatherman 1955:46) and grows when the native vegetation is leafless. Leatherman says (1955:26) that Lonicera japonica growth begins before deciduous trees produce leaves. In Tennessee, it was noted (Leatherman 1955:46) that growth began (in 1953) during the middle of January. In a New Jersey forest, growth began when the temperature was between 1.1° C and 8.9° C (34 and 48° F) (Leatherman 1955:45). The end result is that once L.japonica gets started in an area such as this island where climate, soil, and prairie sod are not limiting factors (see Leatherman 1955:30, 32), it creates and promotes its own best environment, and the forest is ultimately doomed. Leatherman reports (1955:62) that this species blooms profusely in full sun with a heavy production of fruit; therefore, as the forest opens up not only is vegetative growth increased but so is seed production. The potential germination percentage of these is about 85%, while the actual percentage is 63% (Leatherman 1955:63, 38). Penfound describes (1966:189) two treeless, vine-dominated communities: one dominated by Vitis spp. on an island and the other dominated by Ampelopsis arborea (pepper vine) and Smilax bona-nox (bullbrier). Without some other limiting factor, the final result on the disturbed forest areas of Theodore Roosevelt Island may well be a similar treeless community dominated by Lonicera japonica. That other limiting factor may be Hedera helix.
This vine (L. japonica) is not reported as a pest in Japan (Leatherman 1955:29, 64) and there appear to be two possible reasons for this. In a climatic analysis which involved only one Japanese station, the type locality of Nagasaki, Letherman learned (1955:17, 79) that it receives more rainfall (and has a mesothermal rain-forest climate) than areas in the United States where L. japonica is a pest. Secondly, the common horticultural variety in the United States is L. japonica var. halliana (Dipp.) Nichols. (Leatherman 1955:4) which is a more vigorous variant than other members of the species (Leatherman 1955:5). In Japan, the common variety is apparently L. japonica var. repens (Sieb.) Rehd. (Leatherman 1955:15). Through evolution, Japanese forests probably have been selected for resistance to the vine which is confined to its niche.

|
| The author (right) and his assistant, Michael J. Blymyer, working in an upland Hedera helix block. |
Hedera helix
The impact of Hedera helix on the forests of Theodore Roosevelt Island is similar to that of Lonicera japonica in that it destroys the forest.
From general observations on the island, it appeared that light was a limiting factor for H. helix (as well as for L. japonica). Grasovsky (1929:49, 25-27), however, believes, after investigating several species including H. helix, that the effect of light in the forest has been overrated. Grasovsky's study (1929:28, 30. 31-35, 37-39, 40, 41) is the only one found that deals with light as it relates to this exotic vine. A brief review of this investigation as it relates to H. helix will be instructive. The experiment concerning this species consisted of a box fitted with a glass window at one end through which daylight passed. Cuttings of H. helix were placed in pots and two plants were placed at each of four distances from the window. The box was ventilated to control the temperature. Hourly measurements of light were taken on all clear days in the summer for the 10-month experiment. At the end of the experiment, the Hedera helix receiving a maximum illumination of 10,000 foot candles (ft-c) and 280 ft-c were in vigorous condition, while those receiving 65 ft-c were in good condition, and those receiving 25 ft-c were in poor condition. If 10,000 ft-c are considered full sunlight, then 280 ft-c are approximately 3% of full sunlight (Grasovsky 1929:35). In like manner, 65 ft-c and 25 ft-c are about 1% and 0%, respectively. Although those at 65 ft-c were considered in good condition, they were dying. The dry weights of the plants (all species studied) at the end of the experiment were too variable to support the conclusion of more biomass associated with more light; Hedera dry weights are not mentioned specifically. This experiment was apparently not replicated and the potted plants apparently were not weighed before the experiment. Grasovsky also took hourly light measurements at frequent intervals during the growing season on two plots under white pine; the forest floor was covered with pine needles or had a scanty vegetation. Since the light in these pine stands sometimes exceeded the minimum light requirement as found by Grasovsky in previous experiments, he concluded that light was not the limiting factor. On the basis of Toumey's trenched-plot experiments in the same plots, Grasovsky concludes that soil moisture is the factor limiting growth under the pines.
The quantitative data for or against light as a limiting factor for Hedera helix growth in the forest seemed inadequate. I used intact plants already established in the habitat and replicated both my survey observations (in time and space) and my experiments. In addition, the 1 x 1-m plots were trenched to nullify the moisture factor. According to Grasovsky's study, H. helix ought to do well at 3% of full sunlight and the critical point would be somewhere between 3% and 1%. This means that all the forest areas on the island that are presently without H. helix are available habitat waiting for invasion (Tables 51-58) because they all receive much more than 3% of full sunlight. My controlled light experiment with cover (vigor) comes closest to Grasovsky's experiment and it shows (Table 74) that with an increase in shade or decrease in light the condition of H. helix will decline. The situation is more apparent when leaf biomass rather than vigor (cover) is considered (Table 74). For example, some raw data (part of which enters the vigor and light equation of Table 74) show that between 4 and 7% of full sunlight was associated with 25-58 cm2/dm2 of H. helix, while 65 to 68% of full sunlight was associated with 100 cm2/dm2 of H. helix.
It should be evident that the greatest single factor that accounts for Hedera helix growth on the island upland is the amount of sunlight (Table 74; note the coefficient of determination). It is equally evident that light is not as much of a factor in H. helix growth as it is in Lonicera japonica growth. Compare the coefficient of determination for significant regressions of Table 67 with Table 68 and Table 74 with Table 75. There is some other important factor(s) operating. Lack of moisture is probably not the factor limiting the spread of H. helix since its growth in the more moist flood plain was slower than on the upland of the island (Table 6), and yet the light relations of both Hedera habitats were not significantly different (Tables 51-58).
The events that lead to forest destruction by H. helix appear to be similar in some respects to those associated with L. japonica. As with L.japonica, some disturbance of the vertical structure of the forest must occur on the upland areas, for H. helix areas have a different proportion of the vertical layers present than the nonexotic, upland forest (Tables 78, 81; also Table 77). Vertical-structure disturbance allows more light to come into the forest (Tables 51-58). About half the year, mostly in winter and spring, the H. helix upland areas have light intensities similar to the upland forest without exotics.
The situation on the flood plain is different. The flood plain without exotics is normally more open or vertically empty than the island upland forest without exotics (Table 78) and allows more light to enter (Tables 51-58), for about 3/8 of the year (mostly summer and fall). As might be expected, then, the vertical structures on the flood plain with and without Hedera helix are not only similar to each other but similar to the upland areas with H. helix (Tables 77, 78), and the light relations of these three habitats are not significantly different either (Tables 51-58). This does not necessarily mean that the vertical structure on the flood plain is in itself conducive to H. helix invasion without disturbance. The flood plain is not only structurally different from the island upland forest (see also Table 81), but the flood plains with and without exotics are different (Table 80).
There is a peculiarity of vertical structure associated with H. helix, whether on the island upland or on the flood plain, in that some strata are correlated with other strata (Table 80). This correlation does not occur in the other areas studied, exotic or otherwise. On a qualitative basis, this type of correlation began to emerge only when large numbers of observations were made (Table 79).
As pointed out earlier in this discussion, light is an important factor in the growth of Hedera helix on the island. The more light, the heavier the growth of mature or established H. helix (Table 68). The light-stimulated growth influences biomass to a greater extent than cover (vigor, Table 74). Light is less of a factor in growth when H. helix is invading an area (Table 68). Only 41% of the variation in total biomass is explained by referring to percent of open sunlight.
In the disturbed upland forest, H. helix suppresses the normal ground layer and in a sense forms an additional layer at a lower height than the original (Table 77). To this extent, H. helix acts like Lonicera japonica.. But on the flood plain, the ground layer is merely replaced compositionally.
In either case, flood plain or island upland, the differential biomass growth due to sunlight is not significantly different in one habitat over another (Table 2). There may be a slight difference in cover between the two habitats (Table 4), with the upland having the greater cover. The rate at which both cover and biomass is produced does differ (Tables 5, 19). English ivy grows faster on the island upland (Tables 6, 21). One factor which slows the growth on the flood plain is apparently a high water table; no Hedera helix was found growing in the swamp (Table 96) which is often only a few centimeters lower than the flood plain.
Kassas (1952:50. 58, 59, 61) found the same result in studying drainage factors in a fen: H. helix is limited by waterlogging of the soil, and Mittmeyer (1931:367) calls this species a xerophyte. Another related factor is flooding itself, which is not only a more severe case of high water table but is accompanied by hydraulic force against the plants. This slows the rate of growth even more (Tables 5, 7, 22; the 1.25 years data were taken after the flood of Hurricane Agnes).
The flood of Hurricane Agnes (June 1972), although unusually severe in volume (Table 23), would not be different in kind from lesser more frequent floods. Hedera and other ground-cover plants associated with it have no influence on how much mud is deposited in big floods (Tables 24, 23). A combination of mud deposition and water force removed all vegetation to a significant degree from square meter plots. The H. helix was more vulnerable than the native flood-plain vegetation (Tables 25, 26, 27).
Hedera. regardless of habitat, suppresses herbs (Tables 28, 33), but it suppresses them more on the upland (which explains the dearth of wild flowers) than on the flood plain (Tables 28, 33, 38). Reproduction of woody plants in general and trees and other woody plants in particular apparently are not suppressed in either habitat, except for a possibility on the upland (Tables 28, 29, 33, 34, 39, 40, 41). Some data for the upland (Table 28) indicate suppression of nontree, woody plants and possibly trees (the latter group just misses significance), but the other data cited do not necessarily support this suppression. Apparently, the suppression, if it exists on the upland, is subtle and a larger sample would be required for it to be revealed. In any case, herb suppression is greater than woody-plant suppression regardless of habitat (Tables 28, 29, 33, 34).
On the flood plain, the impact of Hedera helix on the herbs appears to be about the same as the impact of the flood of a large hurricane (Tables 27, 33); the impact of the exotic vine on upland herbs exceeds this (Table 38). The woody plants of the ground layer, although affected as much as the herbs by the big flood (Table 27), appear not to be affected at all by flood-plain Hedera vegetation (Table 33). At ground level, there is apparently no competition between H. helix and other woody plants on the flood plain: possibly the same may be said for the island upland (Table 28). But as was previously pointed out, the data are not entirely clear whether H. helix impact is the same on both upland and flood plain of the island. If it now is assumed that there is a slight but real difference in the impact between these two habitats, then there is a theoretical explanation, at least in part. As was brought out in the discussion of light and vertical structure, the flood plain is more open than the upland forest. This relative emptiness is apparently due to the fact that floods periodically remove both herbaceous and woody plants in the ground layer (Table 27), thus the flood plain is a naturally disturbed area. As a disturbed area, it partakes of the characteristics of a density-independent dominated habitat (Gadgil and Solbrig 1972:17, 18, 24, 26). Since the plants, including H. helix, are more subject to density-independent conditions, the impact of this vine (or any other species) is low. However, when the physical disturbances (floods in this case) are removed as, for example, on the upland, then density-dependent factors take over and H. helix would be expected to make a greater impact on the vegetation (Gadgil and Solbrig 1972:15, 16). The woody-plant data were collected after the big flood; had they been collected before, the flood-plain data might have been closer to those of the upland. To that extent, theory explains otherwise anomalous data. More data are needed for drawing more definite conclusions.
Regardless of the impact or degree of impact at the ground layer on woody vegetation by this exotic vine, there is an impact on the overstory trees and by deduction on the other layers as well. It kills them, especially the Ulmus. The situation appears to be as follows, and is based on the same assumption as was made previously with the Lonicera japonica population: if the present percent or proportion of infestation is the same at all stages of the mature tree—alive and healthy, dying, dead standing, fallen dead—then the population is assumed to be stabilized on these trees.
Forty-two percent of the overstory Ulmus americana have Hedera helix growing on them compared to 44% which have neither this vine nor Lonicera japonica (Table 82). This tree species, it will be recalled, is the principal dominant on the island upland. The infestation level on dying Ulmus is about the same as on the healthy Ulmus (Table 83) and it does not differ significantly from the level on all other overstory trees (Tables 83, 84). The infestation level is virtually the same on dead and dying overstory trees except for Ulmus. Dead Ulmus has significantly more H. helix. What has brought this about?
Had H. helix been stimulated by the opening of the crowns due to dying trees as was the case with L. japonica, it would have been tardy in its stimulation compared with L. japonica (Tables 67, 68, 74, 75) and the effect would be realized after, not before, the results of L. japonica stimulation. In other words, the effect of such light stimulation should be reflected on the present generation of healthy trees instead of on a past generation which is reflected on dead standing trees (which in turn predates the dying generation that has a high L. japonica population) (Tables 83, 84).
If Ulmus americana wood were especially durable, like Castanea dentata (chestnut), then the high proportion of dead trees with the exotic vine would merely be due to an accumulation of dead trees that were tardy in falling. But Ulmus is not known for this kind of durability, and its relatively abundant sapwood compared to heartwood argue against this explanation (Wilson and Loomis 1967: 148; Esau 1965:249, 250; Collingwood and Brush 1955:71; Koehler 1949:833, 834; Boyce 1948:448-450).
Another possibility is that Ulmus trees are dying off at a faster rate than they can fall. If Ceratostomella ulmi (Dutch elm disease fungus) were responsible, Lonicera japonica would almost immediately respond and the present generation of Ulmus would have a higher or at least as high a proportion of L. japonica as dying Ulmus. This is not the case; it is lower (Tables 82, 83), and as explained previously with the Lonicera discussion, the effect of C. ulmi has declined from a previous high.
Hedera helix itself appears to be killing the trees, not only Ulmus but other overstory and understory trees as well. The process is merely speeded up on the principal dominant. This conclusion counters the essentially dataless statement of Edlin (1970:56), who says that H. helix probably does not kill a tree, however, if it grows on a tree that dies, it is stimulated in two ways: (1) full light in the dead crown of the tree, and (2) increased nutrients from the soil. Edlin's main point, however, is that broad-leaved evergreens are able to utilize winter sunlight beneath a leafless canopy of tall, broad-leaved trees and this gives such evergreens competitive power. Atanasiu (1965) found, under natural light and temperature conditions, that photosynthesis occurs in H. helix into late autumn up to the first days of December, then in January the plants are below the compensation point. Photosynthesis reappears in February (Atanasiu 1965). Because Theodore Roosevelt Island is closer to the equator than Europe, photosynthesis would not be expected to be as temperature-limited, so photosynthesis in Hedera probably occurs all winter. Since native deciduous vines can destroy native deciduous trees (Penfound 1966:187, 188), it should be expected that an evergreen vine would do the same at an accelerated rate except possibly on Quercus spp. and Fagus grandifolia; these species of Fagaceae retain their dead leaves throughout the winter and would offer some shade deterent to an evergreen vine. As with deciduous vines, the action is apparently to shade out and break the branches of the host tree. As the tree is shaded out, growth is suppressed (Table 96) and the tree dies. This suppression would take place on Ulmus and other deciduous trees as well as on Robinia pseudo-acacia (black locust).
The sequence of events on the island which has led to the killing of overstory trees by this vine appears to be as follows. English ivy apparently was planted around the Mason mansion (see Introduction). This would have been no earlier than about 1792 (Thomas 1963:2, 11, 15; Duhamel 1935:137, 138). As the vine reached the tops of the trees or wall, it would flower in the full sunlight (Edlin 1970:55). The seeds would have been scattered by animals to account for Olmsted and Pope's report (1934:7) that the vine occurred in scattered areas as ground cover and in some of the trees. The young growth as it invades an area is not limited by light (Table 68) and, since it is a tendril liana, it does not have the disadvantage of the twiner such as Lonicera (see also Daubenmire 1965:305, 306). As the evergreen foliage of the vine mingles with the deciduous foliage of the tree, it begins to shade them out, growth of the tree is suppressed, and the increasingly open crown stimulates the vine already present to more luxuriant growth (Tables 68, 74) which further shades out the tree leaves.
When the trees fall either naturally or are cut, the vine increases on the trees (Table 85). The increase is significantly greater on trees other than Ulmus in a natural fall and significantly greater on Ulmus in a cut fail (Tables 85, 88). The explanation of this difference is possibly in the way the tree falls. When a tree dies, it falls apart limb by limb. Perhaps when the trunk finally falls, there are more limbs remaining on non-Ulmus so the opening created is larger than that for Ulmus, but since the Ulmus crown is large and spreading, it opens a larger hole in the canopy when it is cut. In either case, more light is let into the forest and this apparently stimulates Hedera helix growth; and if stimulated enough, it flowers and this increases the seed for further distribution.
Of course, increased light from an opened canopy does not directly stimulate new invasion (Table 68), but the old growth would be stimulated to flower (Edlin 1970:55). In addition, as more H. helix is present, this will be the ground cover which will not show differentiation between fallen logs and soil.
Thirteen percent of the naturally fallen Ulmus trees had Hedera helix growing on them before the fall. This is significantly greater than the 9% of all other naturally fallen trees that also had the vine before their fall (Tables 85, 88). A later generation of trees shows 38% of dying Ulmus and 35% of all other trees with the vine (Table 83). Although Ulmus has the greater percentage, this is not significant (Table 84). The present generation of healthy Ulmus has an even higher percentage (Table 82). The H. helix population, then, has increased over the years and has received direct assistance and a real boost by man when Ulmus is cut (Tables 85. 88).
Not only has the H. helix population increased, but it is taking over areas formerly occupied by Lonicera japonica (see Introduction and Table 96). These two factors, increased abundance and superior competition, help explain the invasion of canopy openings by new growth.
The increased abundance of Hedera helix on the island and on the trees is also killing the trees at a faster rate. It would kill understory trees in the same manner. American elms with H. helix are dying faster than other elms of the same species without H. helix or other trees; the dead trees are accumulating in the upright position (Table 83). The reason, apparently, is that their vigor has been lowered by the presence of Dutch elm disease. Since the fungus causing this disease experienced a high population in the recent past, an increased mortality is to be expected with Ulmus trees that have Hedera growing on them. It seems evident, that in the more remote past, Ulmus also experienced a period of high mortality from H. helix. This is indicated by the significantly higher percentage of downed Ulmus trees with H. helix before they fell naturally than all other downed trees which were infested with Hedera before the fall (Tables 85, 88). This former period of increased mortality on an already steadily rising rate may have been in response to the same factors as the more recent one: cutting of Ulmus, and the subsequent increases in populations of the smaller European engraver beetle, Scolytus multistriatus, and the Dutch elm disease fungus, Ceratostomella ulmi, which reduce tree vigor and allow H. helix to overpower the principal dominant at a faster rate.
English ivy is able to outcompete the herbs and trees (both stratal layers) apparently because it is evergreen and probably grows all winter in this area, while the native vegetation is dormant. It does not create its own best habitat in the sense that Lonicera japonica does; it apparently has relatively little influence on the nonherbaceous ground layer. By killing the trees at an accelerated rate, however, the end result of the forest will be replacement by a Hedera helix-dominated community with few, if any, woody plants getting into the height of the shrub layer. This fate is similar to that described for L. japonica.
Hedera helix has been reported as a pest in its native Europe, but apparently only in disturbed habitats. Wyman (1954:46) says that in Europe it has become a pest and overruns many gardens and that it must be grubbed out in the same way we must frequently remove L. japonica. In succession from a conifer plantation on a drained fen in England, H. helix constituted 77% of the dry crop of the ground layer in an Acer pseudoplatanus (sycamore maple) and Fraxinus excelsior (European ash) woods (Kassas 1952:50, 58).
Hedera also dominates the ground layer in the Fageta hederosa (beech-English ivy) beech forests of Daghestan, Russian Soviet Federated Socialist Republic (Ljvov 1970:1246), is an important member of the ground layer in one of the Fageto—Quercetum (beech—oak) forest types in Bab, Czechoslovakia (Kubicek and Brechtl 1970:27, 34, 35), and in a new association Fraxineto—Quercetum petraeae carpinuloso—hederosum, (ash—sessile oak—horn beam—English ivy) in the Moldavian SSR, the ground cover consists of only this ivy (Geideman and Simonov 1971:84). Europe, in general, and these areas in particular are all farther north than the southeastern United States (Fernald 1950:1078) where Hedera helix has become naturalized. Therefore, in its natural range, temperature is probably an important factor that limits its growth and prevents it from destroying forests. If photosynthesis stops in H. helix for about 2 months in Romania (Atanasiu 1965), then in most of Europe it must stop for a longer period of time. In the southeastern United States, photosynthesis probably seldom stops in Hedera.
Iris pseudacorus
Although Iris pseudacorus is found both close to the tidal gut and the tree line of the swamp-marsh transition, the vegetation type throughout its occurrence is dominated by Peltandra virginica (Table 92). The light relations are fairly uniform throughout this vegetation, with possible exceptions in June, September, and December (Tables 59-66). At these times, the transition areas may receive less light than the open marshes (Tables 60, 64, 66), but this is not clearly related to presence or absence of I. pseudacorus. The consistency with which the transitions receive less light than the open marsh (seven out of eight light measurement times) indicates that there may be a real, although slight, difference between the two because of the presence of the nearby trees, but more investigation would be needed to demonstrate this. Even though the Iris produces more biomass in the swamp-marsh transition than in the open marsh (Table 2), light is not a principal factor (Table 91). There may, however, be a point at which light does become limiting. For example, in the shade on a wet flood plain, Iris seedlings were found but they never became established (Table 96).
The closest to a limiting factor that was found was the length of inundation by water as shown primarily but not entirely by topographic elevation (Tables 89-91; see also narrative Results under Replacement of Exotic Iris). The I. pseudacorus occurs in the higher areas. Although only 47% of the variation in biomass is associated with topographic elevation and hence a shorter duration of inundation by water (Table 91), it was sufficiently accurate to predict that the river level of the Potomac had risen during the growing seasons of the past several years (Table 95). The prediction was verified.
Another factor which limits Iris pseudacorus is Acorus calamus (Tables 49, 50, 96). This factor occurs only in the swamp-marsh transition. From the standpoint of water inundation, I. pseudacorus. in the competition experiments with A. calamus, was probably close to its optimum, i.e., it was not continuously inundated.
In spite of these two factors which tend to limit I. pseudacorus growth, this aquatic species will probably not disappear from the island by itself because of the disturbance of the habitat by man. Its impact on the aquatic ecosystem, although not quite as spectacular as that of Hedera helix and Lonicera japonica on forests, appears, nevertheless, to be considerable. This species apparently speeds up the destruction of the marsh by promoting expansion of the swamp and apparently preempts space and thus reduces the food supply of Aix sponsa (Linnaeus) (Wood Duck) which occurs on the island. The natural range of this duck has decreased over 30% (Martin et al. 1951:4). It has only been since 1941 that this duck generally has increased to the point that limited hunting is allowed (Martin et al. 1951:65). The sequence of events that leads to this putative impact is as follows.
Cody (1961:139, 141) says that, in Canada, the initial escape of Iris pseudacorus from cultivation is probably from rhizomes which have been discarded or washed by floods from low-lying gardens. Subsequent spread comes both from breaking up of the rhizomes or from the abundantly produced seed (Cody 1961:139). Possibly a similar action has taken place here. About 50 years ago, this Iris was, in the Washington, D.C. region, established downstream from the island (Hitchcock and Standley 1919:126). Dyke Marsh, which is located approximately 14.5 km downstream from the island, was noted by McAtee (1918:96) for the abundance of the introduced I. pseudacorus. It is unlikely that the Iris arrived on the island by either tidal action (the seeds do float) or by wild animal carrier. The seeds are rather large to be carried inadvertently on the muddy feet of birds and the species is not listed as a wildlife food (Martin et al. 1951:117, 237; Fassett 1940:347).
It is speculated that this species arrived on Theodore Roosevelt Island by the deliberate dumping or planting near the tidal gut in the vicinity of an old bridge. It is around the piers of this former bridge that the greatest colony in areal extent exists. More of my plots were located here than at any other place in the open marsh. It is of note that one plot was located on top of a sheet of buried rusted metal. For Iris pseudacorus to survive in the wettest part of the marsh, it would have to be raised above the general level of the marsh (Table 90) so that it would not be inundated for very long (Table 91). A lowered river level along with these artificial hummocks is especially conducive to establishment (Table 95). The next largest colony is located in the vicinity of a large Taxodium distichum tree. Bald cypress was planted on the island in the 1930s (Thomas 1963:50). It is known that the transpiration of trees on flat lands lowers the water table (U.S. Forest Service 1954:17). Most of the other areas in the open marsh that were large enough to place plots were associated with trees either native or the introduced T. distichum. The critical point, then, that is going to determine whether I. pseudacorus or Peltandra virginica becomes established in the open marsh is dependent upon the presence of trash (or excessive river debris) or lowered water tables from trees. A lowered river level enhances the establishment and spread, but it should be evident that if the river level drops too far, the Peltandra marsh will disappear by desiccation (Results, Replacement of Exotic Iris). When it comes to establishment by seed, the critical point may be the millimeters difference between the larger Peltandra berry and the smaller Iris seed which apparently germinates only in moist not inundated soil.
Similar factors would operate in the establishment of Iris pseudacorus near the swamp tree line. Here, obviously, transpiration of trees in the growing season becomes a more important factor in lowering the water table and providing a raised support from tree roots. Here, also, because of the minor differences in topography due to the slope toward the gut (Table 89, Fig. 6), a lowered river level becomes even more important in the growth of I. pseudacorus (Table 91). There is another factor that enters into raising the soil level above the water level. The National Park Service has built a gravel trail through the marsh, swamp, and flood plain of the island. The erosion of the trail contributes to filling the marsh and providing habitat for the Iris, and the slopes of the trail side itself form an excellent habitat. Several of my swamp-marsh transition plots were located in the vicinity of this trail.
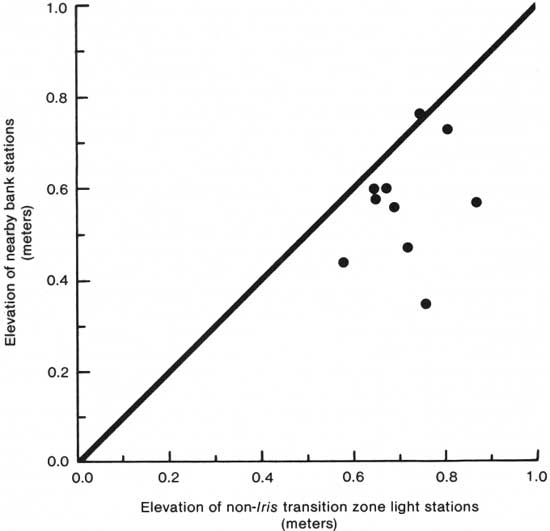
|
| Fig. 6. Comparison of tree line stations with their bank stations in the marsh and swamp—marsh transition zone. |
With the removal of Peltandra virginica habitat and its take-over by I. pseudacorus comes the reduction in food supply for Aix sponsa. Up to 25% of the diet of these ducks may come from the berries of P. virginica (Martin et al. 1951:447), but I. pseudacorus is not eaten (Martin et al. 1951:65). Stewart and Robbins (1958:21) consider this duck to be one of the primary species of breeding birds in the Coastal Plain of Maryland and the District of Columbia. In the Piedmont physiographic province, it is considered a secondary species (Stewart and Robbins 1958:29). The marsh as well as swamp and flood plain of Theodore Roosevelt Island is not only in the Coastal Plain, but is located at the very edge of the Coastal Plain (Thomas 1963:7, 39; the area marked "swamp" includes the flood plain). Aix sponsa males and females have been reported by me and others for this island (NPS, unpubl. data). Theoretically, then, an increase or decrease of an important food at the edge of their primary breeding range might be critical to the abundance of this duck on the island, especially during the nesting season when Peltandra virginica is eaten (Stewart and Robbins 1958:85; Martin et al. 1951:65).
In the swamp-marsh transition area of the marsh there is an additional impact. In the past, the swamp has been advancing on the marsh (Thomas 1963:39, 47), with the normal sequence being that the swamp trees (in this case Fraxinus pennsylvanica) grow up and overtop the Salix (Table 96). Salix caroliniana (Coastal Plain willow) is closely related to S. nigra (Fernald 1950:504) and so is probably as intolerant of shade as S. nigra (Fowells 1965:652). The Salix (S. nigra black willow and S. caroliniana) would die out in the shaded areas of the swamp and be replaced by more shade-tolerant species. The swamp is a five-layered community (Table 76) in which the vertical structure is relatively empty, that is, few layers are represented at any given point (Table 78).
The transition area with Iris pseudacorus is significantly different from the area without (Tables 76, 81; narrative Results, Limiting Factors, Vegetational Strata as a Limiting Factor). The difference shown in the tables is in the woody vegetation under the overstory. The vertical structure of the Iris transition is more like the swamp than the non-Iris transition is like the swamp (Table 78). The qualitative difference between the two transitions is that the non-Iris area has an understory but no shrub layer and the Iris area is just the reverse (Table 76). Another difference is that in the non-Iris transition there is a dependence of the low herb layer on the understory. This correlation ceases to exist in the transition with Iris, and does not exist in the swamp (narrative Results, Limiting Factors, Vegetational Strata as a Limiting Factor).
The mat of Iris rhizomes prevents the germination and seedling development of Salix spp. Salix nigra must have exposed mineral soil for its best development (Fowells 1965:651) and with I. pseudacorus the soil is covered, but with Peltandra virginica, there is much exposed soil available. Giliham (1957:765) found, in a study of coastal vegetation, that I. pseudacorus was one of two chief species that occurs on non-organic soils. Rubtzoff (1959:31) found it growing along the sides of a dirt road which crosses a marsh, and my own observations show (Table 96) that it grows well on gravel. Thus, it appears that it is in very direct competition with Salix nigra on mineral soil. The larger Iris seedling apparently outgrows the Salix even though S. nigra can grow 4 ft in 1 year (Fowells 1965:651), germinates within 24 hours after falling (U. S. Forest Service 1948:328), and has a high germinative capacity (Fowells 1965:651). Apparently, Iris can germinate faster (Table 96) and it grows horizontally over the ground. Black willow has the disadvantage of greatly reduced viability with a few days of dry conditions (Fowells 1965:651). On the other hand, I. pseudacorus seed which was stored dry over winter had a germination percentage of 33 (Table 48). Although this is a low percentage, it is the same that Kartaschoff (1958:151, 152) found under favorable temperatures.
By suppressing the Salix spp. and providing a raised surface, the Iris promotes the invasion of Fraxinus or other trees which do not require a mineral surface. (See Fowells 1965: 185-187 and U. S. Forest Service 1948:181 for F. pennsylvanica seedbed.) Thus, by providing a raised, moist surface rather than an inundated surface, the I. pseudacorus hastens the succession from marsh to swamp. All the transition areas which are not presently occupied by Iris are potential Iris habitat (Table 90).
Iris apparently increases its areal extent by creating new habitat for itself. As the rhizomes grow, they compact the soil so much that a hardpan develops (Table 94). I noticed while removing Iris for the biomass studies that there were often more than one layer of live rhizomes; three layers of live rhizomes were found in two of the transition plots. As the I. pseudacorus grows, it literally squeezes the water out of the soil to create its own drier habitat. In the areas of best Iris growth (the transition), this hardpan is even better developed (Table 94; tetrachoric coefficient +0.92) and results in a definite gleization of the soil (Table 93), which indicates a lack of oxygen (Lutz and Chandler 1946:408). It appears, then, that the area, the transition, where I. pseudacorus has the greatest impact (reduction of the Peltandra virginica marsh both by preempting P. virginica habitat and by promoting swamp invasion), is not only the area where it makes the best growth but also has the greatest potential for creating its own habitat, thus further speeding up the succession of marsh to swamp.
Raven and Thomas (1970) say that, in one place in California, Iris pseudacorus is growing to the complete exclusion of Typha and other characteristic California marsh plants and that it will spread and displace many native plants. They present no data, and perhaps some other factor such as drainage was operating. A detailed study in southern Finland (Perttula 1952) showed that I. pseudacorus with four other species replaced Typha latifolia (common cat-tail) as the first succession after a lake was drained. I found no evidence of Typha being replaced by I. pseudacorus on the island.
There are two mitigating factors which are operating to slow down the accelerated succession which is promoted by the Iris: Acorus calamus and a rising water level.
Acorus calamus produces rhizomes which are smaller in diameter than I. pseudacorus, but the mat which is formed appears to be just as rhizomatous. Acorus is taking over Iris areas in the swamp-marsh transition on the island (Table 96). Acorus can successfully outcompete I. pseudacorus under water inundation levels that appear favorable to Iris (Tables 49, 50), with the result that some wildlife is benefited. Araceous seeds in general are eaten by Aix sponsa (Fassett 1940:344, 352) although A. calamus produces small amounts compared to P. virginica. The larger benefit is with Ondatra zibethicus (Linnaeus), the muskrat. This resident mammal is known to feed on A. calamus (Fassett 1940:344). Iris of other species are only occasionally eaten (Fassett 1940:347; Paradiso 1969:111); the nibbled I. pseudacorus on the island (Table 96) possibly is in the same category. Acorus is not a major food of Ondatra (Paradiso 1969:111; Martin et al. 1951:236), but in the declining population of Maryland (Paradiso 1969:112), this araceous species may be of some importance. The O. zibethicus in the marsh of Theodore Roosevelt Island are unique; they make no dome-shaped lodges, only bank dens. Paradiso (1969:112) states that the dome-shaped structure is made in the marshes, but the bank burrow is made in the banks of streams and other bodies of water.
As mentioned earlier, Acorus calamus produces little seed. I have never seen very many flowers of Acorus in the natural state and have never seen any fruits or seeds. This species puts most of its resources into vegetative structure and hence would be considered a K-strategist, especially when compared with I. pseudacorus (Table 96). Iris pseudacorus is an r-strategist. The implications are, then, that I. pseudacorus will be most successful in a density-independent mortality environment and A. calamus in a density-dependent one (see Gadgil and Solbrig 1972:14, 17-20). Both species are in the emergent zone of marsh vegetation and one might well predict on the basis of r- and K-strategy theory that as the zone fills with vegetation and density-dependent factors be come more important, the r-strategist. I. pseudacorus, will not do as well, which is what is happening. About 50 years ago, A. calamus was common along the lower Potomac which is downstream from Washington, D.C. (Hitchcock and Standley 1919:20, 114). This species either arrived on the island after I. pseudacorus or if it arrived before or at the same time, it survived at a selective disadvantage or only in small areas until the habitat filled up. [The marsh is less than 200 years old (Thomas 1963:46, 47)]. The presence of I. pseudacorus probably did not hasten the filling process, it apparently substituted for Peltandra virginica as discussed earlier. This would indicate that Acorus would eventually take over Peltandra areas also since P. virginica is an r-strategist compared with A. calamus. The important difference, however, appears to be that with the substitution (I. pseudacorus), the marsh supports less wildlife, succession is short-circuited, and the marsh is shorter-lived as discussed previously.
The other mitigating factor in the growth and spread of the Iris is the rise in the water level of the river which has taken place for about the last decade (Table 95). As shown previously, I. pseudacorus decreases as length of water inundation increases (Table 91). Since the Iris near the tidal gut is at a lower elevation, it is affected first and has virtually disappeared (Figs. 3, 4; Tables 44, 46) and the Peltandra has begun to take over the I. pseudacorus areas (Figs. 3, 5; Tables 45, 47). The tree-line area was affected later with the same pattern of Iris decline and Peltandra increase (Figs. 2, 4, 5; Tables 42, 43, 46, 47). It is expected that I. pseudacorus will almost disappear in the transition area. Peltandra in the open marsh did not invade or respond as quickly to Iris decline as it did in the swamp-marsh transition. This would follow from the greater topographic differential between the exotic and nonexotic in the two marsh areas (Table 90) because it would be more difficult to float seed to the higher elevation because the velocity of runoff is greater with higher elevations (Gilluly et al. 1955:133).
There will, however, be certain refugia, areas of higher elevation, that may be scattered around the marsh. Most of these refugia will be the result of man's activities. For example, the largest refuge is the side of the gravel trail which coincides with the tree line of the marsh-swamp transition. From these refuges, the Iris will spread again when the water level of the river begins to recede and the tidal inundation therefore is shortened. If the Iris creates new habitat for itself as suggested, then the refugia will increase in area.
Comparisons of the Three Exotic Species
There is a similarity of circumstances associated with each of these three exotic species: disturbed habitats. Even in a successional stage, disturbance appears to be the key to entry. An important facet of the disturbances is that they are products of man's activity, and they have resulted in biological explosions on the island in the sense that Elton (1958:15) describes for larger areas. One of the greatest impacts of man on the environment is his introduction of exotic species into environments that he has disturbed. These introductions often trigger a sequence of events that counter the goals or best interests of man himself. Theodore Roosevelt Island is an example where the planned and unplanned activities of man, both before and since the area became a park, have set in motion biological forces which are destined, unless countered, to destroy the upland and flood-plain forests and the marshes.
It is to be expected that different organisms might have different degrees of impact on a given environment. The same organism in closely related environments may have widely differing impacts. On the basis of resources taken out of circulation in the form of dry biomass, the three exotic species in the six microhabitats present four grades of impact, with Iris pseudacorus in the transition having the greatest and Lonicera japonica in a mildly disturbed forest (natural understory), the least (Table 2). The fact that an herb has a greater control of the environment than a woody plant should not be too surprising since the herb in question, I. pseudacorus, is in the tall herb layer of its vegetation type which has only four layers (Table 76), while the woody vines, as studied, are in a subordinate layer in their type which has five layers.
Hedera helix and L. japonica live in similar habitats. The mildly disturbed forest with L. japonica is identical with the upland H. helix forest in the light intensity that reaches the forest floor (Tables 51-58). In vertical structure, they are the same in number of layers (Tables 76, 77), in the relative emptiness of the layers (Table 78), and in the relative variability of the depth of the woody layers (Table 81). There is, however, one aspect of vertical structure where they differ. Forests with H. helix have a significant correlation of one of the woody layers with another woody layer, whereas this is not the case with L. japonica (Table 80). Since, in the case of the upland H. helix forest, the correlation is between overstory and understory trees, and since it is only rather recently that H. helix has been extremely abundant, this correlation is probably not the result of H. helix impact, but probably a condition conducive to Hedera invasion. Lonicera which has been abundant on the island for a long time (Thomas 1963:49), was apparently more abundant than H. helix until recently (Introduction), and has generally a greater suppression of trees especially in much disturbed habitats, is possibly the creator of the correlations that are found both in the upland and flood-plain Hedera forests.
The fall of any canopy tree in the forest, from any cause, increases the abundance of both exotic vines, but not equally (Table 85). Natural falls of trees other than Ulmus americana promote Hedera helix more than a natural fall of Ulmus, but Ulmus which is cut down stimulates growth of Hedera more than other trees which are cut.
The increased presence of these two exotic vines with fallen trees appears to be due mostly to the increase in light. Both species respond to an increase in light or a decrease in shade (Tables 67, 68, 74, 75), but they do not respond equally. Lonicera is more dependent upon light than H. helix, and in Lonicera this dependence is greater with new growth than old growth (Table 67), but the reverse is true of Hedera (Table 68). In Hedera, chlorophyll A formation takes place at a faster rate than leaf biomass (Table 74). Climax dominants in layered vegetation must be shade-tolerant while young (Daubenmire 1965:229, 230; Oosting 1956:92). H. helix appears to behave like a climax dominant.
Hedera not only has a greater biomass per square meter than Lonicera japonica regardless of habitat (Table 2), but in mildly disturbed upland forests, it grows faster per unit of time (Tables 5, 6, 7, 96). The impact of the biomass, however, differs between the vines and the life forms that they suppress. For example, in mildly disturbed forests, H. helix suppresses herbs more than L. japonica does (Table 38), and the tendency is for L. japonica to suppress trees more than H. helix under the same disturbance conditions (Table 40). Under severe disturbance conditions, L.japonica definitely suppresses trees in the ground layer more than any H. helix on the island. Woody plants other than trees are suppressed about equally by the exotic vines (Table 41). Biologically, the two exotic vines combined suppress the reproduction of Podophyllum peltatum (Table 32), but the increase after release was not great enough to be statistically significant. This is probably due to the low number of replications available.
With Lonicera japonica suppressing woody plants, particularly trees, and growing over shrubs and small trees and killing them, and Hedera helix killing the larger trees and suppressing herbs, the upland and flood plain forests are slowly disappearing and will be replaced by a vine-dominated community. Hedera has taken over L. japonica areas (Table 96 and Introduction) and is able to accumulate biomass faster than Lonicera. The final community will be dominated by Hedera helix. The only forests on the island will be those of the swamp which will have increased in size due to the destruction of the marsh by Iris pseudacorus.
| <<< Previous | <<< Contents >>> | Next >>> |
13/chap4.htm
Last Updated: 08-Oct-2008